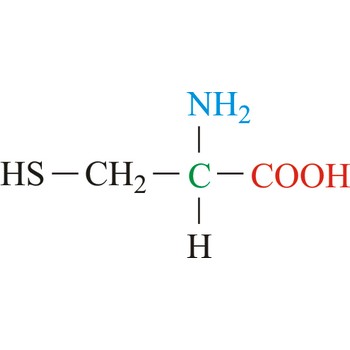
cysteine → cistein
Cysteine is neutral amino acids with polar side chains. Because of its high reactivity, the thiol group of cysteine has numerous biological functions. It serves as a potent nucleophile and metal ligand (particularly for iron and zinc), but is best known for its ability to form disulfide bonds, which often make an important contribution to the stability of extracellular proteins. Cysteine is a non-essential amino acid, which means that it is biosynthesized in humans.
- Abbreviations: Cys, C
- IUPAC name: 2-amino-3-sulfanylpropanoic acid
- Molecular formula: C3H7NO2S
- Molecular weight: 121.16 g/mol
Mannich reaction → Mannichova reakcija
Mannich reaction is a process in which hydrogen atoms in organic compounds are replaced with a methyl group.
monobasic acid → monobazična kiselina
Monobasic acids are acids that have only one replacable hydrogen atom per molecule (HCl, HNO3).
diagenesis → dijageneza
Diagenesis is the process that turns sediments into sedimentary rocks. The lithification (literally turning into stone) of the sediments is usually accomplished by a cementing agent. How the weight of the overlying material increases the grains closer together, reducing pore space and eliminating some of the contained water. This water may carry mineral components in solution, and these constituents precipitate as new minerals in the pore spaces. This causes cementation, which will then start to bind the individual particles together. Further compaction and burial may cause recrystallization of the minerals to make the rock even harder.
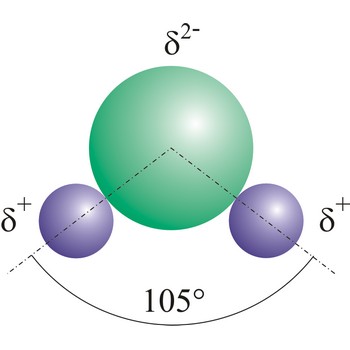
dipole molecule → dipolna molekula
Dipole molecules are created when mutual electronic pair at covalent bond is asymmetrical. If different atoms are bonded by a covalent bond, which can have different electron affinity, then the the atom with greater electron affinity will attract the electron pairs more strongly. In this way an asymmetrical distribution of negative charge appears in a molecule, so one part of the molecule becomes relatively negatively (the one closer to the electron pair) and the other becomes relatively positively charged.
monodentate ligand → monodentantni ligand
Monodentate ligand is a ligand that has only one atom that coordinates directly to the central atom in a complex. For example, ammonia and chloride ion are monodentate ligands of copper in the complexes [Cu(NH3)6]2+ and [CuCl6]2+.
neutral substance → neutralna tvar
Neutral substance is a substance that shows no acid or base properties, has an equal number of hydrogen and hydroxyl ions and does not change the colour of litmus-paper.
dipole moment → dipolni moment
Electric dipole moment (μ) is a product of the positive charge and the distance between the charges. Dipole moments are often stated in debyes; The SI unit is the coulomb metre. In a diatomic molecule, such as HCl, the dipole moment is a measure of the polar nature of the bond; i.e. the extent to which the average electron charges are displaced towards one atom (in the case of HCl, the electrons are attracted towards the more electronegative chlorine atom). In a polyatomic molecule, the dipole moment is the vector sum of the dipole moments of the individual bonds. In a symmetrical molecule, such as tetrafluoromethane (CF4) there is no overall dipole moment, although the individual C-F bonds are polar.
Citing this page:
Generalic, Eni. "Vodikova veza." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table