carbon → ugljik
Carbon has been known since ancient times. The origin of the name comes from the Latin word carbo meaning charcoal. Graphite form of carbon is a black, odourless, slippery solid. Graphite sublimes at 3825 °C. Diamond form is a clear or colored; an extremely hard solid. C60 is Buckminsterfullerine. Carbon black burns readily with oxidants. Carbon is made by burning organic compounds with insufficient oxygen. There are close to ten million known carbon compounds, many thousands of which are vital to organic and life processes. Radiocarbon dating uses the carbon-14 isotope to date old objects.
cycloalkanes → cikloalkani
Cycloalkanes are cyclic saturated hydrocarbons containing a ring of carbon atoms joined by single bonds. They have the general formula CnH2n, for example cyclohexane, C6H12. In general, they behave like the alkanes but are rather less reactive.
dehydrogenation → dehidrogenacija
Dehydrogenation is a chemical reaction in which hydrogen is removed from a compound. Dehydrogenation of organic compounds converts single carbon-carbon bonds into double bonds. It is usually affected by means of a metal catalyst or in biological systems by enzyme dehydrogenases.
cement → cement
Cement is any various substances used for bonding or setting to a hard material. Portland cement is a mixture of calcium silicates and aluminates made by heating limestone (CaCO3) with clay (containing aluminosilicates) in a kiln. The product is ground to a fine powder. When mixed with water it settles in a few hours and then hardens over a longer period of time due to the formation of hydrated aluminates and silicates.
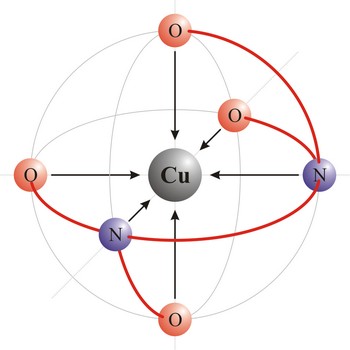
chelate → kelat
Chelate is a compound characterized by the presence of bonds from two or more bonding sites within the same ligand to a central metal atom. For example, copper complexes with EDTA to form a chelate. Chelate complexes are more stable than complexes with the corresponding monodentate ligands.
deuterium lamp → deuterijeva svjetiljka
Deuterium or hydrogen lamp is used as a source of continuous radiation in UV part of the spectrum.
diazo compounds → diazo-spojevi
Diazo compounds are compounds having the divalent diazo group, =N+=N-, attached to a carbon atom. The term includes azo compounds, diazonium compounds, and also such compounds as diazomethane, CH2=N2.
electron pair → elektronski par
Electron pair is two electrons within one orbital with opposite spins responsible for a chemical bond.
equivalent weight → ekvivalentna masa
Equivalent weight of a substance participating in a neutralization reaction is that mass of substance (molecule, ion, or paired ion) that either reacts with or supplies 1 mol of hydrogen ions in that reaction.
Equivalent weight of a substance participating in an oxidation/reduction reaction is that weight which directly or indirectly produces or consumes 1 mol of electrons.
Citing this page:
Generalic, Eni. "Vodikova veza." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table