living planet index → indeks života na planetu
The Living Planet Index (LPI) reflects changes in the health of the planet's ecosystems by tracking trends in nearly 8000 populations of vertebrate species. The LPI first calculates the annual rate of change for each species population in the dataset, then calculates the average change across all populations for each year from 1970, when data collection began, to 2007, the latest date for which data is available.
The Global LPI shows a decline of around 30 % from 1970 to 2007, based on 7953 populations of 2544 species of birds, mammals, amphibians, reptiles and fish.
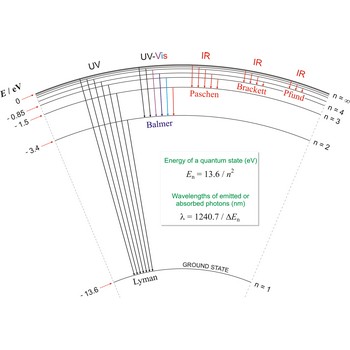
Lyman series → Lymanova serija
Lyman series is the series of lines in the spectrum of the hydrogen atom which corresponds to transitions between the ground state (principal quantum number n = 1) and successive excited states.
magnesium → magnezij
Magnesium was discovered by Sir Humphry Davy (England) in 1808. The origin of the name comes from the Greek word Magnesia, a district of Thessaly. It is lightweight, malleable, silvery-white metal. Burns in air with a brilliant white flame and reacts with water as temperature elevates. Can ignite in air. React violently with oxidants. Magnesium is found in large deposits in the form of magnesite, dolomite and other minerals. It is usually obtained by electrolysis of melted magnesium chloride (MgCl2) derived from brines, wells and sea water. Used in alloys to make airplanes, missiles and other uses for light metals. Have structural properties similar to aluminium.
manganese → mangan
Manganese was discovered by Johann Gahn (Sweden) in 1774. The origin of the name comes from the Latin word magnes meaning magnet, or magnesia nigri meaning black magnesia (MnO2). It is hard, brittle, grey-white metal with a pinkish tinge. Impure forms are reactive. Rusts like iron in moist air. Manganese is most abundant ores are pyrolusite (MnO2), psilomelane [(Ba,H2O)2Mn5O10] and rhodochrosite (MnCO3). Pure metal produced by mixing MnO2 with powered Al and ignited in a furnace. Used in steel, batteries and ceramics. The steel in railroad tracks can contain as much as 1.2 % manganese. It is crucial to the effectiveness of vitamin B1.
mercury → živa
Mercury has been known since ancient times. The origin of the name comes from the Latin word hydrargyrum meaning liquid silver. It is heavy, silver-white metal, liquid at ordinary temperatures. Stable in air and water. Unreactive with alkalis and most acids. Gives off poisonous vapour. Chronic cumulative effects. Mercury only rarely occurs free in nature. The chief ore is cinnabar or mercury sulfide (HgS). Used in thermometers, barometers and batteries. Also used in electrical switches and mercury-vapour lighting products.
metal → metal
Metals are materials in which the highest occupied energy band (conduction band) is only partially filled with electrons.
Their physical properties generally include:
- They are good conductors of heat and electricity. The electrical conductivity of metals generally decreases with temperature.
- They are malleable and ductile in their solid state.
- They show metallic lustre.
- They are opaque.
- They have high density.
- They are solids (except mercury)
- They have a crystal structure in which each atom is surrounded by eight to twelve near neighbours
Their chemical properties generally are:
- They have one to four valence electrons.
- They have low ionisation potentials; they readily lose electrons.
- They are good reducing agents.
- They have hydroxides which are bases or amphoteric.
- They are electropositive.
Metallic characteristics of the elements decrease and non-metallic characteristics increase with the increase of valence electrons. Also metallic characteristics increase with the number of electron shells. Therefore, there is no sharp dividing line between the metals and non-metals.
Of the 114 elements now known, only 17 show primarily non-metallic characteristics, 7 others are metalloids, and 89 may be classed as metals.
metallic glass → metalno staklo
Certain alloys can solidify by extremely rapid cooling out of melt without formation of a crystal lattice, that is in the amorphous form - such, amorphous alloys are so called metallic glasses. The alloy of zirconium, beryllium, titanium, copper, and nickel is one of the first metallic glasses that can be made in bulk and formed into strong, hard, useful objects.
Unlike pure metals and most metal alloys, metallic glasses have no regular crystalline structure. This lack of long range order or microstructure is related to such desirable features as strength and low damping which is one reason why the premier use for zirconium-based metallic glass is in the manufacture of expensive golf club heads. Metallic glasses can be quite strong yet highly elastic, and they can also be quite tough (resistant to fracture). Even more interesting are the thermal properties; for instance, just like an oxide glass, there is a temperature (called the glass transition temperature) above which a metallic glass becomes quite soft and flows easily. This means that there are lots of opportunities for easily forming metallic glasses into complex shapes.
mutarotation → mutarotacija
Mutarotation is the change in optical rotation accompanying epimerization. In carbohydrate chemistry this term usually refers to epimerization at the hemiacetal carbon atom. In general α- and β-form are stable solids, but in solution they rapidly equilibrate. For example, D-glucose exists in an equilibrium mixture of 36 % α-D-glucopyranose and 64 % β-D-glucopyranose, with only a tiny fraction in the open-chain form. The equilibration occurs via the ring opening of the cyclic sugar at the anomeric center with the acyclic form as the intermediate. Mutarotation was discovered by French chemist Augustin-Pierre Dubrunfaut (1797-1881) in 1846.
neodymium → neodimij
Neodymium was discovered by Carl F. Auer von Welsbach (Austria) in 1885. The origin of the name comes from the Greek words neos didymos meaning new twin. It is silvery-white, rare-earth metal that oxidizes easily in air. Reacts slowly in cold water, more rapidly as heated. Metal ignites and burns readily. Neodymium is made from electrolysis of its halide salts, which are made from monazite sand. Used in making artificial ruby for lasers. Also in ceramics and for a special lens with praseodymium. Also to produce bright purple glass and special glass that filters infrared radiation. Misch metal, used in the manufacture of pyrophoric alloys for cigarette lighters, contains about 18 % neodymium metal. (Typically composition of misch metal are Ce:Nd:Pr:La:Other rare earth=50:18:6:22:4). Neodymium is used to create some of the most powerful permanent magnets on Earth, known as NIB magnets they consist of neodymium, iron, and boron.
non-metal → nemetal
Non-metals are defined as elements that are not metals.
Their physical properties generally include:
- They are poor conductors.
- They are brittle, not ductile in their solid state.
- They show no metallic lustre.
- They may be transparent or translucent.
- They have low density.
- They form molecules which consists of atoms covalently bonded; the noble gases are monoatomic.
Their chemical properties are generally:
- They usually have four to eight valence electrons.
- They have high electron affinities (except the noble gases)
- They are good oxidising agents (except the noble gases)
- They have hydroxides which are acidic (except the noble gases)
- They are electronegative.
Citing this page:
Generalic, Eni. "Voda." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table