nitrogen → dušik
Nitrogen was discovered by Daniel Rutherford (Scotland) in 1772. The origin of the name comes from the Greek words nitron genes meaning nitre and forming and the Latin word nitrum (nitre is a common name for potassium nitrate, KNO3). It is colourless, odourless, generally inert gas. Minimally reactive at room temperature. A component of many organic and inorganic compounds. Makes up about 78 % of earth’s atmosphere. Nitrogen is obtained from liquid air by fractional distillation. Primarily to produce ammonia and other fertilizers. Also used in making nitric acid, which is used in explosives. Also used in welding and enhanced oil recovery.
non-metal → nemetal
Non-metals are defined as elements that are not metals.
Their physical properties generally include:
- They are poor conductors.
- They are brittle, not ductile in their solid state.
- They show no metallic lustre.
- They may be transparent or translucent.
- They have low density.
- They form molecules which consists of atoms covalently bonded; the noble gases are monoatomic.
Their chemical properties are generally:
- They usually have four to eight valence electrons.
- They have high electron affinities (except the noble gases)
- They are good oxidising agents (except the noble gases)
- They have hydroxides which are acidic (except the noble gases)
- They are electronegative.
nonpolar molecule → nepolarna molekula
Nonpolar molecule is a molecule which has no separation of charge, so no positive or negative poles are formed. For example, the Cl2 molecule has no polar bonds (molecule with one type of atom), CH4 is a non-polar molecule (due to its symmetry). Nonpolar molecules do not dissolve in water as they cannot form hydrogen bonds (thus are hydrophobic) but do dissolve in lipids or fats (lipophilic).
nuclear magnetic resonance → nuklearna magnetska rezonancija
Nuclear magnetic resonance (NMR) is a type of radio-frequency spectroscopy based on the magnetic field generated by the spinning of electrically charged atomic nuclei. This nuclear magnetic field is caused to interact with a very large (1 T - 5 T) magnetic field of the instrument magnet. NMR techniques have been applied to studies of electron densities and chemical bonding and have become a fundamental research tool for structure determinations in organic chemistry.
octahedral molecular geometry → oktaedarska geometrija molekule
Octahedral molecular geometry (square bipyramidal shape) describes the shape of compounds where six atoms or ligands are symmetrically arranged around a central atom. The sulfur hexafluoride (SF6), with six bonding pairs, is predicted and found to be a regular octahedron. Four of the attachments are positioned in a square plane with 90° bond angles. The remaining two attachments are positioned perpendicular (90°) to the square plane at opposite ends of the central atom. Molecules with an octahedral electron pair geometries have sp3d2 (or d2sp3) hybridization at the central atom.
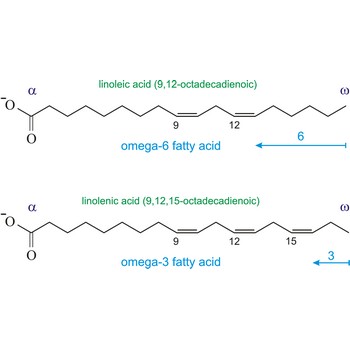
omega-3 fatty acids → omega-3 masne kiseline
Omega-3 fatty acids are polyunsaturated fatty acids, meaning they contain more than one double bond. The name omega-3 indicates that the first double bond occurs on the third carbon atom (n-3) from the methyl (-CH3) end of the molecule (omega position). The three main omega-3 fatty acids are alpha-linolenic acid (ALA, 18:3n-3), eicosapentaenoic acid (EPA, 20:5n-3), and docosahexaenoic acid (DHA, 22:6n-3). ALA comes from plants. EPA and DHA come from fish.
Similarly, the first double bond in omega-6 fatty acids is located between the sixth and seventh carbon atom (n-6) from the methyl end of the fatty acid (omega end).
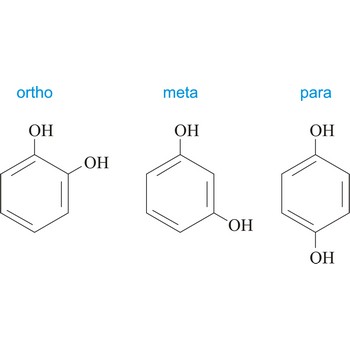
ortho position → ortho položaj
Ortho position in organic chemistry is the one in which there are two same functional groups, tied to a ring of benzene in the positions 1 and 2. The abbreviation o- is used, for example, o-Hydroquinone is 1,2-dihydroxybenzene.
oxidation number → oksidacijski broj
Atoms can give one or more electrons for bond forming. The valence of any atom, which comes from stechiometrical relation of interbonded atoms, is called an oxidation number or an oxidation degree. Oxidation number of atoms in elementary state is zero. An atom of greater electronegativity has a negative, and an atom of lesser electronegativity has a positive oxidation number.
para position → para položaj
Para position in organic chemistry is the one in which there are two same functional groups tied to a ring of benzene in the position 1 and 4. The abbreviation p- is used, for example, p-Hydroquinone is 1,4-dihydroxybenzene.
Citing this page:
Generalic, Eni. "Trostruka veza." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table