electronegativity → elektronegativnost
Electronegativity is a parameter originally introduced by L. Pauling which describes, on a relative basis, the power of an atom to attract electrons. For example, in hydrogen chloride, the chlorine atom is more electronegative than the hydrogen and the molecule is polar, with a negative charge on the chlorine atom.
There are various ways of assigning values for the electronegativity of an element. Pauling electronegativities are based on bond dissociation energies using a scale in which fluorine, the most electronegative element, has the value 4 and francium, the lowest electronegative element, has the value 0.7.
enzyme → enzim
Enzyme is a protein that acts as a catalyst in biochemical reactions. Each enzyme is specific to a particular reaction or a group of similar reactions. Many require the association of certain nonprotein cofactors in order to function. The molecule undergoing a reaction (the substrate) binds to a specific active site on the enzyme molecule to form a short-lived intermediate: this greatly increases (by a factor of up to 1020) the rate at which the reaction proceeds to form the product.
epoxy resin → epoksi smola
Epoxy resins are thermosetting resins produced by copolymerising epoxide compounds with phenols (e.g. epichlorohydrin and bisphenol A). They contain ether linkages (-O-) and form a tight, cross-linked polymer network. Toughness, good adhesion, corrosive-chemical resistance, and good dielectric properties characterise epoxy resins. Most epoxy resins are two-part types which harden when blended.
Fajans’ rules → Fajansova pravila
Fajans’ rules, formulated by American chemist of Polish origin. Kazimierz Fajans (1887-1975), indicating the extent to which an ionic bond has covalent character caused by polarisation of the ions. Covalent character is more likely if:
1. the charge of the ions is high;
2. the positive ion is small or the negative ion is large;
3. the positive ion has an outer electron configuration that is not a noble- gas configuration.
solvation → solvatacija
Solvation is the process by which solvent molecules surround and interact with solute ions or molecules.
terminal → terminalni
Terminal in chemistry means: the end of a polymer molecule and a point at which electron connections can easily be made or broken.
tertiary alcohol → tercijarni alkohol
Tertiary alcohols are aliphatic alcohols in which the hydroxyl group (-OH) is attached to a tertiary carbon atom.
thermodynamics → termodinamika
Thermodynamics is the scientific study of the interconversion of heat and other forms of energy.
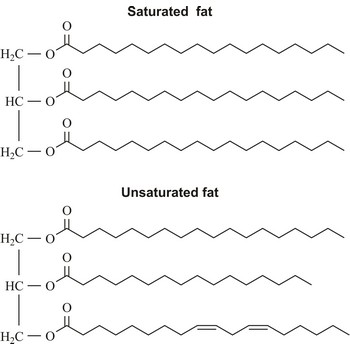
fat → mast
Fats are esters of glycerol and long chain carboxylic acids. Fats occur widely in plants and animals as a means of storing food energy, having twice the calorific value of carbohydrates. Fats derived from plants and fish generally have a greater proportion of unsaturated fatty acids than those from mammals. Fats may be either solid or liquid at room temperature, depending on their structure and composition. Unsaturated fats are liquid at room temperature.
Plant oils may be hardened by the addition of hydrogen atoms, converting double bonds to single bonds. This process is known as hydrogenation. Hydrogenated vegetable oils are often present in margarine and other processed foods.
Alkali hydrolysis of fat with sodium hydroxide it gives glycerol and soap (i.e. a mixture of the sodium salts of the fatty acids).
Citing this page:
Generalic, Eni. "Trostruka veza." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table