Newton’s gravitational law → Newtonov zakon gravitacije
Every object in the universe attracts every other object with a force (gravitational force FG) directed along the line through centres of the two objects that is proportional to the product of their masses and inversely proportional to the square of the distance between them.
m1 and m2 are masses of the two objects and r is the distance between them. G is universal constant of gravitation, which equals 6.67•10-26 N m2 kg-2. Strictly speaking, this law applies only to objects that can be considered pointlike object. Otherwise, the force has to be found by integrating the forces between various mass elements.
It is more properly to express Newton’s gravitational law by vector equation:
in which r1 and r2 are position vectors of masses m1 and m2.
Gravitational forces act on distance. Newton’s gravitational law is derived from Kepler’s law for planetary motion, using a physical assumption considering Sun as the centre and the source of gravitational force.
Additionally, every object moves in the direction of the force acting on it, with acceleration that is inversely proportional to the mass of object. For bodies on the surface of Earth, the distance r in gravitational law formula is practically equal to the Earth radius, RE. If the mass of the body on Earth surface is m and the mass of earth is ME, the gravitational force acting on that body can be expressed as:
where g is gravitational acceleration which is, although dependent on geographical latitude, usually considered as constant equal to 9.81 m s-2.
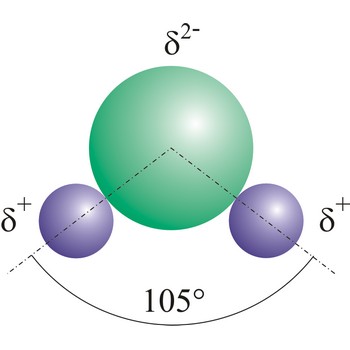
polar molecule → polarna molekula
Polar molecules are molecules at which centres of gravity of positive and negative charge are not in the same point.
position vector → položajni vektor
The location of a point-like object relative to the origin of a coordinate system is given by a position vector r, which in unit vector notation is
where x, y and z are the scalar components of r.
potentiometric titration → potenciometrijska titracija
Potentiometric titration is a volumetric method in which the potential between two electrodes is measured (referent and indicator electrode) as a function of the added reagent volume. Types of potentiometric titrations for the determination of analytes in photoprocessing solutions include acid-base, redox, precipitation, and complexometric.
Potentiometric titrations are preferred to manual titrations, since they are more accurate and precise. They are also more easily adapted to automation, where automated titration systems can process larger volumes of samples with minimal analyst involvement.
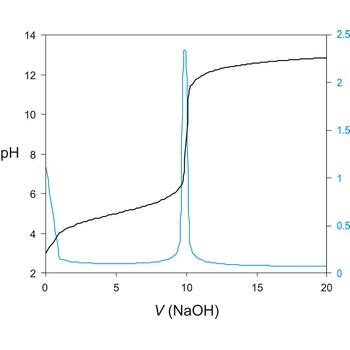
A titration curve has a characteristic sigmoid curve. The part of the curve that has the maximum change marks the equivalence point of the titration. The first derivative, ΔE/ΔV, is the slope of the curve, and the endpoint occurs at the volume, V', where ΔE/ΔV has the maximum value.
retardation factor → faktor zaostajanja
Retardation factor, RF, (in planar chromatography) is a ratio of the distance travelled by the centre of the spot to the distance simultaneously travelled by the mobile phase:
The RF value is characteristic for any given compound on the same stationary phase using the same mobile phase for development of the plates. Hence, known RF values can be compared to those of unknown substances to aid in their identifications.
rotational inertia → moment tromosti
Rotational inertia of a body is defined as
for a system of discrete particles (each of mass mi), and as
for a body with continuously distributed mass (dm is the mass element). ri and r represent the perpendicular distance from the axis of rotation to the mass element of the body.
SI unit for rotational inertia is kg m2.
superfluid helium → superfluidni helij
Superfluidity in helium-4 was discovered in 1938 by the Soviet physicist Pyotr Leonidovich Kapitsa. Helium-4 exhibits superfluidity when it is cooled below 2.18 K (-270.97 C), which is called the lambda (λ) point. At these temperatures, helium-4 exhibits the characteristics of two distinct fluids, one of which appears to flow without friction. An extensive series of experiments showed that in this state of helium, called helium II (He II), there is an apparent enormous rise in heat conductivity, at an increase rate of about three million. Another unusual property of He II is its mobile, rapid flow through capillaries or over the rim of its containment vessel as a thin film that exhibits no measurable viscosity and appears unaffected by the forces of gravity or evaporation and condensation.
titration curve → titracijska krivulja
Titration curve is a graphic representation of the amount of a species present vs. volume of solution added during a titration. A titration curve has a characteristic sigmoid curve. The inflection point in the titration curve marks the end-point of the titration. Blue line is the first derivative of the titration curve.
unequal-arm balance → vaga s različitim krakovima
The lever principle on which these scales are constructed is based on the law of physics that at equilibrium the force applied at one end of the lever multiplied by the length of the arm (distance from the fulcrum to the point where the force is applied) must be equal to the product of the force acting at the opposite end of the lever and the length of the other arm.
The unequal-arm balance is preferred for work when large amounts are to be weighed.
Citing this page:
Generalic, Eni. "Trojna točka." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table