orbital → orbitala
Orbital is the area in space about an atom or molecule in which the probability of finding an electron is greatest.
The possible atomic orbitals correspond to subshells of the atom. Thus there is one s-orbital for each shell (orbital quantum number l = 0). There are three p-orbitals (corresponding to the three values of l) and five d-orbitals. The shapes of orbitals depend on the value of l.
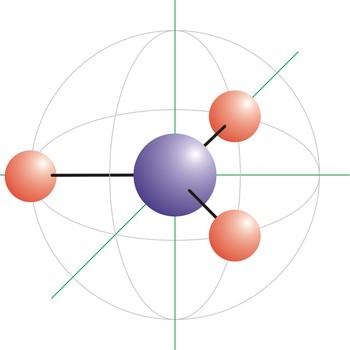
planary structure → planarna struktura molekule
Planary structure of molecule is a structure of molecule in which all atoms in the molecule lie in the same plane.
plastic → plastika
Plastic is a material that can be shaped by the application of heat or pressure. Most are based on synthetic polymers although some are the product of natural substances (such as cellulose derivatives, but excluding the rubbers.). They are usually light and permanent solids, being also heat and electric isolators. If the materials soften again when reheated, they are said to be thermoplastic. If, after fashioning, they resist further applications of heat, they are said to be thermoset.
potential energy → potencijalna energija
Potential energy (Ep) is the energy stored in a body or system as a consequence of its position, shape, or state (this includes gravitation energy, electrical energy, nuclear energy, and chemical energy). Gravitational potential energy is the energy associated with the state of separation between bodies that attracts each other via gravitational force. Elastic potential energy is the energy associated with the state of compression or extension of an elastic object. Thermal energy is associated with the random motions of atoms and molecules in a body.
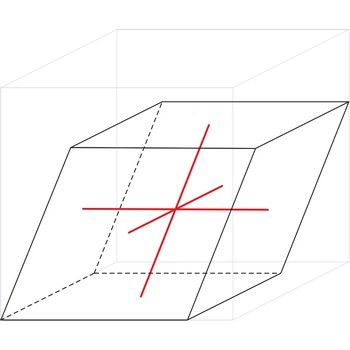
rhombohedral crystal system → romboedarski kristalni sustav
Rhombohedral crystal system is also known as the trigonal system. The crystallographic axes used in this system are of equal length. None of the axes are perpendicular to any other axis.
a = b = c
α= β = γ ≠ 90°
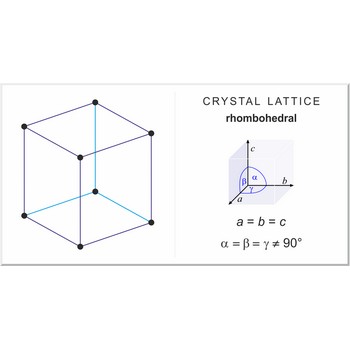
rhombohedral lattice → romboedarska rešetka
Rhombohedral (or trigonal) lattice has one lattice point at the each corner of the unit cell. It has unit cell vectors a=b=c and interaxial angles α=β=γ≠90°.
solid state → čvrsto agregatno stanje
Solid state is characterised by a constant shape and volume. Particles are placed very close to one another and have efect one on another with great attraction forces. Solid bodies do not assume the shape of the container in which they are put.
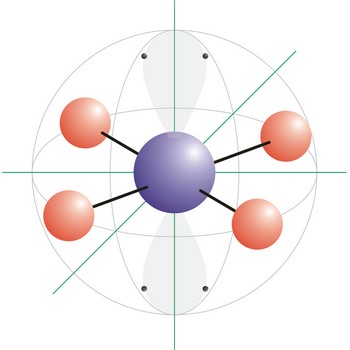
square planar molecular geometry → kvadratna planarna geometrija molekule
Square planar is a molecular shape that results when there are four bonds and two lone pairs on the central atom in the molecule. An example of a square planar molecule is xenon tetrafluoride (XeF4). This molecule is made up of six equally spaced sp3d2 (or d2sp3) hybrid orbitals arranged at 90° angles. The shape of the orbitals is octahedral. Two orbitals contain lone pairs of electrons on opposite sides of the central atom. The remaining four atoms connected to the central atom give the molecule a square planar shape.
standard deviation → standardna devijacija
Standard deviation (σ) is a measure of the dispersion of a set of data from its mean. Standard deviation is a statistical term that measures the amount of variability or dispersion around an average
Suppose there are many measurements of a quantity presumed to be similar, like the size of peas in a pod. If the number of readings for each size were plotted, a bell-shaped curve would probably result, with a few small and large peas and most clustered around the average size. Around two-thirds of all measurements fall in the range spanned by the standard deviation, a measure of the spread.
tetrahedral molecular geometry → tetraedarska geometrija molekule
Tetrahedral is a molecular shape that results when there are four bonds and no lone pairs around the central atom in the molecule. The atoms bonded to the central atom lie at the corners of a tetrahedron with 109.5° angles between them. Molecules with an tetrahedral electron pair geometries have sp3 hybridization at the central atom. The ammonium ion (NH4+) and methane (CH4) have a tetrahedral molecular geometry.
Citing this page:
Generalic, Eni. "Trigonal pyramidal%0Amolecular shape." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table