Results 1–3 of 3 for tetrahedral
tetrahedral molecular geometry → tetraedarska geometrija molekule
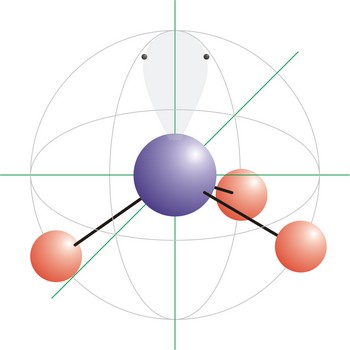
Tetrahedral is a molecular shape that results when there are four bonds and no lone pairs around the central atom in the molecule. The atoms bonded to the central atom lie at the corners of a tetrahedron with 109.5° angles between them. Molecules with an tetrahedral electron pair geometries have sp3 hybridization at the central atom. The ammonium ion (NH4+) and methane (CH4) have a tetrahedral molecular geometry.
allotrope → alotrop
Allotropes are the elements which exist in two or more different forms in the same physical state. Allotropes generally differ in physical properties and may also differ in chemical activity.
Diamond, graphite and fullerenes are three allotropes of the element carbon. Graphite is a soft, black, slippery substance; by contrast, diamond is one of the hardest substances known. The different properties of the allotropes arise from their chemical structures. Diamonds typically crystallize in the cubic crystal system and consist of tetrahedrally bonded carbon atoms. Graphite crystallizes in the hexagonal system. In the fullerenes, the carbon atoms taking the form of a hollow sphere, ellipsoid, or tube.
In some cases, the allotropes are stable over a temperature range, with a definite transition point at which one changes into the other. For instance, tin has two allotropes: white (metallic) tin stable above 13.2 °C and grey (nonmetallic) tin stable below 13.2 °C.
The term allotropes may also be used to refer to the molecular forms of an element. Ozone is a chemically active triatomic allotrope of the element oxygen.
trigonal pyramidal molecular geometry → trigonska piramidalna geometrija molekule
Trigonal pyramidal is a molecular shape that results when there are three bonds and one lone pair on the central atom in the molecule. Molecules with an tetrahedral electron pair geometries have sp3 hybridization at the central atom. Ammonia (NH3) is a trigonal pyramidal molecule.
Citing this page:
Generalic, Eni. "Tetrahedral." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table