effervescence → pjenušanje
Effervescence is the formation of gas bubbles in a liquid by a chemical reaction. An example of effervescence is the release of carbon dioxide which bubbles as a gas from the liquid when limestone chips, which are composed of calcium carbonate, are added to dilute hydrochloric acid.
Carnot cycle → Carnotov kružni proces
Carnot cycle is the most efficient cycle of operations for a reversible heat engine. Published in 1824 by French physicist Nicolas Léonard Sadi Carnot (1796-1832), it consists of four operations on the working substance in the engine:
1-2: Isothermal expansion at thermodynamic temperature T1 with heat QH taken in.
2-3: Adiabatic expansion with a fall of temperature to T2.
3-4: Isothermal compression at temperature T2 with heat QC given out.
4-1: Adiabatic compression at temperature back to T1.
According to the Carnot principle, the efficiency of any reversible heat engine depends only on the temperature range through which it works, rather than the properties of the working substances.
cellulose → celuloza
Cellulose, (C6H10O5)n, is a polysaccharide that consists of a long unbranched chain of glucose units linked by (1→4)-β-glycoside bonds. Nature uses cellulose primarily as a structural material to impart strength and rigidity to plants. Leaves, grasses, and cotton are primarily cellulose. The fibrous nature of extracted cellulose has led to its use in textile industry for the production of cotton, artificial silk, etc. Cellulose also serves as raw material for the manufacture of cellulose acetate, known commercially as acetate rayon, and cellulose nitrate, known as guncotton. Gunncotton is the major ingredient in smokeless powder, the explosive propellant used in artillery shells and in ammunition for firearms.
elution → eluacija
Elution is the process of removing an adsorbed material (adsorbate) from an adsorbent with a liquid (eluent). The solution consisting of the adsorbate dissolved in the eluent is the eluate. Elution is the process used to wash components of a mixture through a chromatography column.
eutectic → eutektik
Eutectic is a solid solution consisting of two or more substances and having the lowest freezing point of any possible mixture of these components.
Eutectic point is the lowest temperature at which the eutectic mixture can exist in a liquid phase. A liquid having the eutectic composition will freeze at a single temperature without a change of composition.
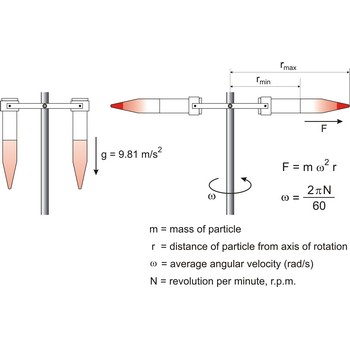
centrifuge → centrifuga
Centrifuge is a device in which solid or liquid particles of different densities are separated by rotating them in a tube in a horizontal circle. The dense particles tend to move along the length of the tube to a greater radius of rotation, displacing the lighter particles to the other end.
chemical equation → kemijska jednadžba
Chemical equation is a way of denoting a chemical reaction using the symbol for the participating particles (atoms, molecules, ions, etc.); for example,
The single arrow is used for an irreversible reaction; double arrows are used for reversible reactions. When reactions involve different phases, it is usual to put the phase in brackets after the symbol.
| s | = | solid |
| l | = | liquid |
| g | = | gas |
| aq | = | aqueous |
The numbers a, b, c, and d, showing the relative numbers of molecules reacting, are called the stoichiometric coefficients. The convention is that stoichiometric coefficients are positive for reactants and negative for products. If the sum of the coefficients is zero, the equation is balanced.
evaporation → isparavanje
Evaporation is the change of state of a liquid into a vapour at a temperature below the boiling point of the liquid.
Citing this page:
Generalic, Eni. "Tekuće agregatno stanje." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table