mutarotation → mutarotacija
Mutarotation is the change in optical rotation accompanying epimerization. In carbohydrate chemistry this term usually refers to epimerization at the hemiacetal carbon atom. In general α- and β-form are stable solids, but in solution they rapidly equilibrate. For example, D-glucose exists in an equilibrium mixture of 36 % α-D-glucopyranose and 64 % β-D-glucopyranose, with only a tiny fraction in the open-chain form. The equilibration occurs via the ring opening of the cyclic sugar at the anomeric center with the acyclic form as the intermediate. Mutarotation was discovered by French chemist Augustin-Pierre Dubrunfaut (1797-1881) in 1846.
optically active matter → optički aktivna tvar
Optically active matter, by polarized light passing through it, turns the flat of polarized light leftwards or rightwards.
phosphorus → fosfor
Phosphorus was discovered by Hennig Brandt (Germany) in 1669. The origin of the name comes from the Greek word phosphoros meaning bringer of light. White phosphorus is white to yellow soft, waxy phosphorescent solid with acrid fumes. Toxic by inhalation, ingestion, or skin contact. Red phosphorus is powdery, non-flammable and non-toxic. Phosphorus is found most often in phosphate rock. Pure form is obtained by heating a mixture of phosphate rock, coke and silica to about 1450 °C. Used in the production of fertilizers and detergents. Some is used in fireworks, safety matches and incendiary weapons. Phosphorus is also important in the production of steels, phosphor bronze and many other products.
photochemical reaction → fotokemijska reakcija
Photochemical reactions are those reactions which are conducted under the influence of light that is under the influence of ultraviolet, visible and infrared part of the light spectrum. Some systems can be influenced only by radiation that is absorbed by that system. Photochemical reactions are for example photosynthesis, creation of photography, generation of phosgene, creation of hydrochloride etc.
photoelectric effect → fotoelektrični efekt
Photoelectric effect is the complete absorption of a photon by a solid with the emission of an electron. The energy of a photon (hν) is
polarimeter → polarimetar
Polarimeter is an appliance which measures the angle of turning of polarisation light.
polarimetry → polarimetrija
Polarimetry measures the overall turning of the flat of polarised light. It is used when analysing optically active substances and compounds.
polonium → polonij
Polonium was discovered by Marie Curie (Poland) in 1898. Named for Poland, native country of Marie Curie. It is silvery-grey, extremely rare, radioactive metal. Soluble in dilute acids. Highly toxic. Severe radiotoxicity. Carcinogen. Polonium occurs in pitchblende. Produced by bombarding bismuth with neutrons. Used in industrial equipment that eliminates static electricity caused by such processes as rolling paper, wire and sheet metal.
promethium → prometij
Promethium was discovered by J. A. Marinsky, Lawrence Glendenin and Charles D. Coryell (USA) in 1945. Named after Prometheus in Greek mythology, who stole fire from the gods. It is rare earth metal of synthetic origin on the earth, naturally made in stars. Poison. Radiotoxic. Radioactive. Promethium does not occur naturally. Found among fission products of uranium, thorium and plutonium. It has been used as a source of radioactivity for thickness-measuring gages.
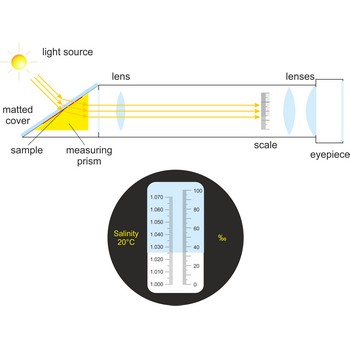
refractometer → refraktometar
Refractometer is an optical device used from measurement of refractive index. A refractometer takes advantage of the fact that light bends as it passes through different materials. It can be used to measure the salinity of water or the amount of sugar in fresh grapes. Refractometers are available with or without automatic temperature compensation (ATC).
When using a conventional saltwater refractometer, a sample is placed on an optical prism in the sample window. As light shines through the sample, it is bent according to the salinity of the water, and casts a shadow on the scale that is visible through the eyepiece. Salinity is read directly through the eyepiece.
Citing this page:
Generalic, Eni. "Svjetlost." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table