mole → mol
Mole (mol) is the SI base unit of amount of substance.
The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kg of carbon 12.
When the mole is used, the elementary entities must be specified and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles. In this definition, it is understood that the carbon 12 atoms are unbound, at rest and in their ground state.
Nernst’s division law → Nernstov zakon razdjeljenja
Nernst’s division law states that a substance is divided between two solvents in a way that proportion of concentrations of that substance is at certain temperatures constant, under the condition that both solvents are in the same molecular state. Division coefficient is a proportion of substance concentration in solvents A i B at a defined temperature.
Appearance of division is used for substance extraction.
solution → otopina
Solutions are homogeneous mixtures of clean substances. Solutions contain two or more substances mixed in a state of molecular dispersion. Component which is found in solution in greater amount than other components is called a solvent and other components are called dissolved substances. Solution can be unsaturated, saturated and oversaturated.
supersaturated solution → prezasićena otopina
Solution is supersaturated when it contains greater quantity of dissolved substance in itself than it corresponds to solubility of that substance at that temperature. It is said to be in an unstable state, and by shaking the vessel containing that such a solution separation of salt surplus can occur.
non-metal → nemetal
Non-metals are defined as elements that are not metals.
Their physical properties generally include:
- They are poor conductors.
- They are brittle, not ductile in their solid state.
- They show no metallic lustre.
- They may be transparent or translucent.
- They have low density.
- They form molecules which consists of atoms covalently bonded; the noble gases are monoatomic.
Their chemical properties are generally:
- They usually have four to eight valence electrons.
- They have high electron affinities (except the noble gases)
- They are good oxidising agents (except the noble gases)
- They have hydroxides which are acidic (except the noble gases)
- They are electronegative.
octet rule → pravilo okteta
Octet rule states that the chemical properties of the elements repeat on a regular basis with increasing atomic mass, and that the chemical properties of each eight element are similar. Since the inert gases, with the exception of helium have eight electrons in their outer shells, this stable electronic configuration is called the octet rule. In chemical reactions atoms of elements tend to react in such a way as to achieve the electronic configuration of the inert gas nearest to them in the periodic table. There are a number of exceptions to the octet rule.
oxidation number → oksidacijski broj
Atoms can give one or more electrons for bond forming. The valence of any atom, which comes from stechiometrical relation of interbonded atoms, is called an oxidation number or an oxidation degree. Oxidation number of atoms in elementary state is zero. An atom of greater electronegativity has a negative, and an atom of lesser electronegativity has a positive oxidation number.
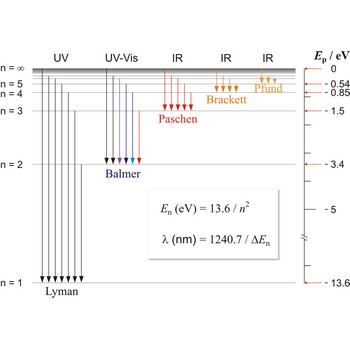
Paschen series → Paschenova serija
Paschen series are the series of lines in the spectrum of the hydrogen atom which corresponds to transitions between the state with principal quantum number n = 3 and successive higher states.
Citing this page:
Generalic, Eni. "States of matter." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table