luminescence → luminiscencija
Luminescence (from Latin lumen, light) is the emission of electromagnetic radiation (UV, visible or IR) from atoms or molecules as a result of the transition of an electronically excited state to a lower energy state, usually the ground state. Luminescence can be divided into categories by duration (fluorescence or phosphorescence) or by the mechanism that creates the light (radioluminescence, electroluminescence, photoluminescence, thermoluminescence, triboluminescence, chemiluminescence, bioluminescence). The prefix identifies the energy source responsible for generating or releasing the light.
Phosphorescence is emission of light from a substance exposed to radiation and persisting as an afterglow after the source of excitation has been removed. Fluorescence, on the other hand, is an almost instantaneous effect, ending within about 10-8 second after excitation.
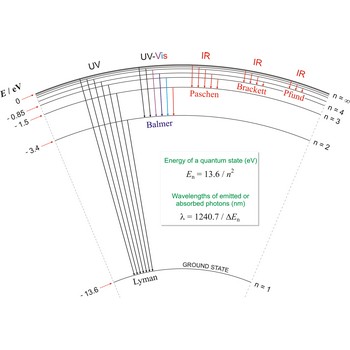
Lyman series → Lymanova serija
Lyman series is the series of lines in the spectrum of the hydrogen atom which corresponds to transitions between the ground state (principal quantum number n = 1) and successive excited states.
mass → masa
Mass (m) is the quantity of matter contained in a particle or body regardless of its location in the universe. Mass is constant, whereas weight is affected by the distance of a body from the centre of the Earth (or of other planet). The SI unit is kilogram.
According to the Einstein equation
all forms of energy possess a mass equivalent.
melting point → talište
Melting point is the temperature at which a solid becomes a liquid at normal atmospheric pressure.
A more specific definition of melting point (or freezing point) is the temperature at which the solid and liquid phases of a substance are in equilibrium at a specified pressure (normally taken to be atmospheric unless stated otherwise). A pure substance under standard condition of pressure has a single reproducible melting point. The terms melting point and freezing point are often used interchangeably, depending on whether the substance is being heated or cooled.
radiation → radijacija
The process of heat transfer which occurs through empty space and can also occur in matter, in the form of electro-magnetic (EM) waves, is called radiation or radiant heat. Whenever EM radiation is emitted and then absorbed, heat is transferred. This principle is used in microwave ovens, laser cutting, and RF hair removal.
regeneration → regeneracija
Regeneration is the process of restoring an ion exchange medium to a usable state after exhaustion. The cation exchanger is normally regenerated with hydrochloric acid and the anion exchanger with sodium hydroxide.
metal → metal
Metals are materials in which the highest occupied energy band (conduction band) is only partially filled with electrons.
Their physical properties generally include:
- They are good conductors of heat and electricity. The electrical conductivity of metals generally decreases with temperature.
- They are malleable and ductile in their solid state.
- They show metallic lustre.
- They are opaque.
- They have high density.
- They are solids (except mercury)
- They have a crystal structure in which each atom is surrounded by eight to twelve near neighbours
Their chemical properties generally are:
- They have one to four valence electrons.
- They have low ionisation potentials; they readily lose electrons.
- They are good reducing agents.
- They have hydroxides which are bases or amphoteric.
- They are electropositive.
Metallic characteristics of the elements decrease and non-metallic characteristics increase with the increase of valence electrons. Also metallic characteristics increase with the number of electron shells. Therefore, there is no sharp dividing line between the metals and non-metals.
Of the 114 elements now known, only 17 show primarily non-metallic characteristics, 7 others are metalloids, and 89 may be classed as metals.
Citing this page:
Generalic, Eni. "States of matter." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table