lead-acid battery → olovni akumulator
Lead-acid battery is a electrical storage device that uses a reversible chemical reaction to store energy. It was invented in 1859 by French physicist Gaston Planté. Lead-acid batteries are composed of a lead(IV) oxide cathode, a sponge metallic lead anode and a sulphuric acid solution electrolyte.
In charging, the electrical energy supplied to the battery is changed to chemical energy and stored. The chemical reaction during recharge is normally written:
In discharging, the chemical energy stored in the battery is changed to electrical energy. During discharge, lead sulfate (PbSO4) is formed on both the positive and negative plates. The chemical reaction during discharge is normally written:
Lead acid batteries are low cost, robust, tolerant to abuse, tried and tested. For higher power applications with intermittent loads however, they are generally too big and heavy and they suffer from a shorter cycle life.
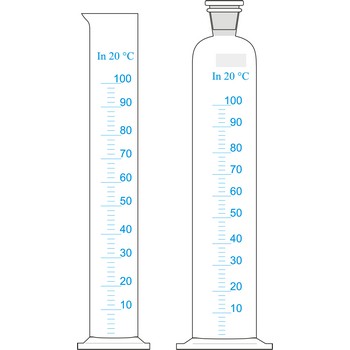
measuring cylinder → menzura
Measuring cylinders (graduated cylinders) are graduated glass cylinders with a capacity from 2 mL to 2 L. They are used when reagent solutions for volume measuring are prepared when accuracy is not of great relevance. The larger the measuring cylinder, the bigger the measuring error.
niobium → niobij
Niobium was discovered by Charles Hatchett (England) in 1801. The origin of the name comes from the Greek word Niobe meaning daughter of Tantalus in Greek mythology (tantalum is closely related to niobium in the periodic table). It is shiny white, soft, ductile metal. Exposed surfaces form oxide film. Niobium occurs in a mineral columbite. It is used in stainless steel alloys for nuclear reactors, jets and missiles. Used as an alloy with iron and nickel. It can be used in nuclear reactors and is known to be superconductive when alloyed with tin, aluminium or zirconium.
Petri dish → Petrijeva zdjelica
Petri dish is a shallow glass or plastic flat bottomed dish with a lid. Used primarily in laboratories for the culture of bacteria and other microorganisms on specially prepared media. It was named after the German bacteriologist Julius Richard Petri (1852-1921) who invented it in 1877.
photomultiplier → fotomultiplikator
Photomultiplier (photomultiplier tube or PMT) is a very versatile and sensitive detector of radiant energy in the ultraviolet, visible, and near infrared regions of the electromagnetic spectrum. A typical photomultiplier tube consists of a photoemissive cathode (photocathode) followed by focusing electrodes, an electron multiplier (dynode) and an electron collector (anode) in a vacuum tube.
picnometer → piknometar
Picnometer is a special glass flask which is used for determining a relative density of liquids using the weight of a known volume. It usually has a glass faceted cork which is pierced in the centre like a thin capillary through which surplus of liquid runs out.
pipette → pipeta
Pipettes are glass tubes which are tapers towards at both ends into narrow opened tubes. According to their design two types of pipettes can be distinguished:
Volumetric pipettes
Volumetric pipettes (transfer or belly pipette) are used in volumetric analysis, when there is a need for taking exact smaller volume of a sample solution or reagent. The upper tube of volumetric pipette has a ringlike marking (mark) which marks its calibrated volume. Pipettes calibrated to deliver (TD or Ex) the indicated volume. By sucking in (with mouth, propipette or a water pump) the liquid is pulled in a little bit above the mark and the opening of the pipet is closed with a forefingertip. Outer wall of pipet is wiped and, with a slight forefinger loosening, the liquid is released until it reaches the mark. Mark must figure as a tangent on a lower edge of the liquid meniscus. A pipette is emptied out by lifting the forefinger off and letting the liquid flow out of the pipette freely. After another 15 s and the tip of the pipette is pulled onto the inner wall of the vessel. It is absolutely forbidden to blow out the contents of the pipette
Graduated pipettes
Graduated pipettes (Mohr pipette) have a scale divided into units of one and of 1/10th of a millilitre. Because of their wide necks it is less accurate than the volumetric pipette. They are used when taking volume of solutions in which accuracy does not have to be very high. They are filled in the same way as volumetric ones and liquid can be gradually released.
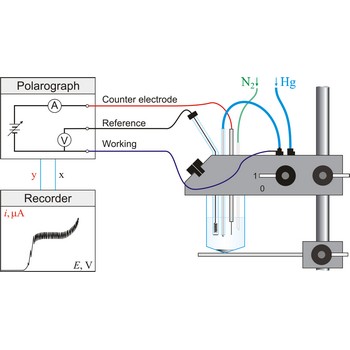
polarography → polarografija
Polarography is a volumetric technique which is based on a diffusion controlled analyte travel to the surface of dropping mercury electrode (DME). The surface of the working electrode (dropping mercury electrode) is constantly renewed under dropping conditions and, thus, the conditions under which reaction takes place are readily reproducible. Depolarisation potential enables identification of ions present in the solution, and by measuring the diffusion current their concentration is calculated. Polarography was discovered in 1922 by the Czech chemist Jaroslav Heyrovský (1890-1967).
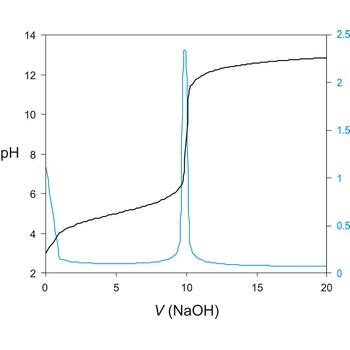
potentiometric titration → potenciometrijska titracija
Potentiometric titration is a volumetric method in which the potential between two electrodes is measured (referent and indicator electrode) as a function of the added reagent volume. Types of potentiometric titrations for the determination of analytes in photoprocessing solutions include acid-base, redox, precipitation, and complexometric.
Potentiometric titrations are preferred to manual titrations, since they are more accurate and precise. They are also more easily adapted to automation, where automated titration systems can process larger volumes of samples with minimal analyst involvement.
A titration curve has a characteristic sigmoid curve. The part of the curve that has the maximum change marks the equivalence point of the titration. The first derivative, ΔE/ΔV, is the slope of the curve, and the endpoint occurs at the volume, V', where ΔE/ΔV has the maximum value.
redox potential → redoks potencijal
Redox potential is the potential of a reversible oxidation-reduction electrode measured with respect to a reference electrode, corrected to the hydrogen electrode, in a given electrolyte.
Citing this page:
Generalic, Eni. "Staklena elektroda." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table