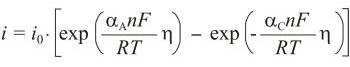Faradaic reaction → Faradejska reakcija
Faradaic reaction is a heterogeneous charge-transfer reaction occurring at the surface of an electrode.
irreversible galvanic cell → nepovrativi galvanski članak
Irreversible galvanic cell is a chemical source of direct current, in which reactions that take place on the electrodes are irreversible.
Bunsen, Robert Wilhem → Bunsen, Robert Wilhem
Robert Wilhem Bunsen (1811-1899) is a German chemist who held professorships at Kassel, Marburg and Heidelberg. His early researches on organometallic compound of arsenic cost him an eye in an explosion. Bunsen's most important work was in developing several techniques used in separating, identifying, and measuring various chemical substances. He also improvement chemical battery for use in isolating quantities of pure metals - Bunsen battery.
The essential piece of laboratory equipment that has immortalized the name of Bunsen was not invented by him. Bunsen improved the burner's design, which had been invented by Faraday, to aid his endeavors in spectroscopy. Use of the Bunsen burner in conjunction with a glass prism led to the development of the spectroscope in collaboration with the German physicist Gustav Kirchoff and to the spectroscopic discovery of the elements rubidium (1860) and cesium (1861).
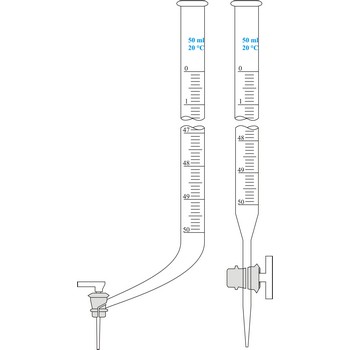
burette → bireta
Burette is a graded glass pipe which on its lower side has a glass faucet by which it can drop a precise quantity of liquid. Inner diameter of a burette must be equal in its whole length, because the accuracy of volume measurement depends upon that. Burettes are primarily used in volumetric analysis for titration with standard solution reagent. Most often Schellbach’s burette is used, graded on 50 mL with division of scale on 0.1 mL. Every burette is calibrated on discharge. For serial determining automatic burettes are used.
Butler-Volmer equation → Butler-Volmerova jednadžba
Butler-Volmer equation is an activation controlled reaction, the one for which the rate of reaction is controlled solely by the rate of the electrochemical charge transfer process, which is in turn an activation-controlled process. This gives rise to kinetics that are described by the Butler-Volmer equation:
where io is exchange current density, η is overpotential (η = E - Eo), n is number of electrons, αA is anodic transfer coefficient, and αC is cathodic transfer coefficient
negative pole → negativan pol
Negative pole is that half-cell in electrochemical cell that has the most negative electrode potential.
overpotential → prenapon
Overpotential (η) is a potential that must be applied in an electrolytic cell in addition to the theoretical potential required to liberate a given substance at an electrode. The value depends on the electrode material and on the current density.
positive pole → pozitivni pol
Positive pole is that half-cell in the electrochemical cell which has the most positive electrode potential.
Citing this page:
Generalic, Eni. "Staklena elektroda." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table