refractive index → indeks loma
For a non-absorbing medium, refractive index (n) is the ratio of the velocity of electromagnetic radiation (light) in vacuum to the phase velocity of radiation of a specified frequency in the medium.
absorbance → apsorbancija
Absorbance (A) is a logarithm of the ratio of incident radiant power (Po) to transmitted radiant power (P) through a sample (excluding the effects on cell walls).
The absorption of light by a substance in a solution can be described mathematically by the Beer-Lambert law
where A is the absorbance at a given wavelength of light, ε is the molar absorbtivity or extinction coefficient (L mol-1 cm-1), unique to each molecule and varying with wavelength, b is the length of light path through the sample (cm), and c is the concentration of the compound in solution (mol L-1).
absorbed dose → apsorbirana doza
For any ionising radiation, absorbed dose (D) is the mean energy imparted to an element of irradiated matter divided by the mass of that element.
absorptance → apsorptancija
Absorptance (α) is the ratio of the radiant or luminous flux in a given spectral interval absorbed in a medium to that of the incident radiation. Also called absorption factor.
alpha particle → alfa-čestica
Alpha particle is a helium nucleus emitted spontaneously from radioactive elements, both natural and manufactured. Its energy is in range 4-8 MeV and is dissipated in a very short path, i.e. a few centimetres of air or less than 0.005 mm of aluminium. As helium nucleus consists of two protons and two neutrons bound together as a stable entity the loss of an alpha particle involves a decrease in nucleon number of 4 and decrease of 2 in the atomic number, e.g.
A stream of alpha particles is known as an alpha ray or alpha-radiation.
blackbody → crno tijelo
In radiation physics, an ideal blackbody is a theoretical object that absorbs all the radiant energy falling upon it and emits it in the form of thermal radiation. Planck’s radiation law gives the power radiated by a unit area of blackbody, and the Stefan-Boltzman law expresses the total power radiated.
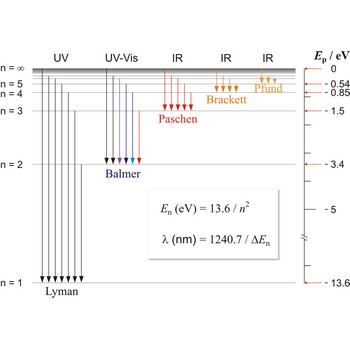
Balmer series → Balmerova serija
Balmer series, Balmer lines is a series of lines in the emission spectrum of hydrogen that involve transitions to the n=2 state from states with n>2.
Bohr atom → Bohrov atom
Bohr atom is a model of the atom that explains emission and absorption of radiation as transitions between stationary electronic states in which the electron orbits the nucleus at a definite distance. The Bohr model violates the Heisenberg uncertainty principle since it postulates definite paths and moment for electrons as they move around the nucleus. Modern theories usually use atomic orbitals to describe the behaviour of electrons in atoms.
deuterium lamp → deuterijeva svjetiljka
Deuterium or hydrogen lamp is used as a source of continuous radiation in UV part of the spectrum.
Citing this page:
Generalic, Eni. "Spektar elektromagnetskog zračenja." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table