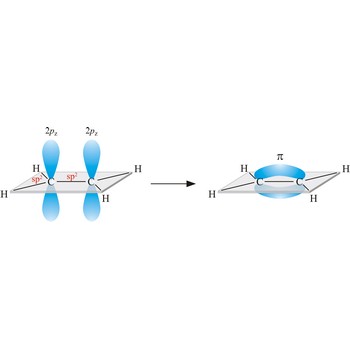
valence bond → valentna veza
In the valence bond theory, a valence bond is a chemical bond formed by overlap of half-filled atomic orbitals on two different atoms.
valence bond theory → teorija valentne veze
Valence bond theory is a theory that explains the shapes of molecules in terms of overlaps between half-filled atomic orbitals, or half filled hybridised orbitals.
square planar molecular geometry → kvadratna planarna geometrija molekule
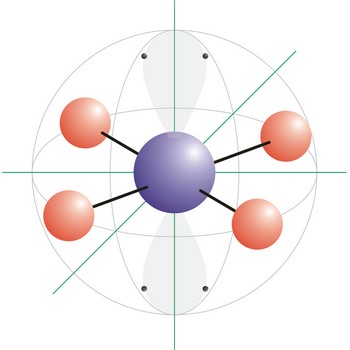
Square planar is a molecular shape that results when there are four bonds and two lone pairs on the central atom in the molecule. An example of a square planar molecule is xenon tetrafluoride (XeF4). This molecule is made up of six equally spaced sp3d2 (or d2sp3) hybrid orbitals arranged at 90° angles. The shape of the orbitals is octahedral. Two orbitals contain lone pairs of electrons on opposite sides of the central atom. The remaining four atoms connected to the central atom give the molecule a square planar shape.
square pyramidal molecular geometry → kvadratna piramidalna geometrija molekule
Square pyramidal is a molecular shape that results when there are five bonds and one lone pair on the central atom in the molecule. Bromine pentafluoride (BrF5) has the geometry of a square pyramid, with fluorine atoms occupying five vertices, one of which is above the plane of the other four. This molecule is made up of six equally spaced sp3d2 (or d2sp3) hybrid orbitals arranged at 90° angles. The shape of the orbitals is octahedral. Because of the high symmetry of the octahedral arrangement, all six positions are equivalent, so it does not matter in which position in the drawing we put the lone pair. The remaining four atoms connected to the central atom give the molecule a square planar shape.
Citing this page:
Generalic, Eni. "Sp hibridna orbitala." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table