covalentity → kovalentnost
Covalentity is a maximum number of covalent bond that one atom can make, it is equal to the number of hydrogen atoms that are combined with other atoms. It is usually constant.
decomposing → raščinjavanje
Decomposing in analytical chemistry means that a certain substance is converted, by melting it with a suitable melting medium (sodium carbonate, sodium hydroxide, sodium peroxide, ...) in the kind of compound which will afterwards that dissolve in water, acid or base very easily.
Biot-Savart law → Biot-Savartov zakon
The magnetic field B due to a current-carrying conductor can be determined by Biot-Savart law. The contribution to magnetic field set up at distance r by the current element IdL is given by expression:
where μ0 is permeability constant. It plays a role in magnetic problems equivalent to the role of permittivity constant μ0 in electrostatics problems. In order to obtain B, contributions of all current elements have to be integrated. In case of a long straight conductor, carrying current I, Biot-Savart law gives:
SI unit for magnetic field B is tesla (T).
Permaeability constant μ0 has value 4π×10-7 T m A-1.
bismuth → bizmut
Bismuth was discovered by Claude Geoffroy (France) in 1753. The origin of the name comes from the German words Weisse Masse meaning white mass; now spelled wismut and bisemutum. It is hard, brittle, steel-grey metal with a pink tint. Stable in oxygen and water. Dissolves in concentrated nitric acid. Bismuth can be found free in nature and in minerals like bismuthine (Bi2S3) and in bismuth ochre (Bi2O3) Main use is in pharmaceuticals and low melting point alloys used as fuses.
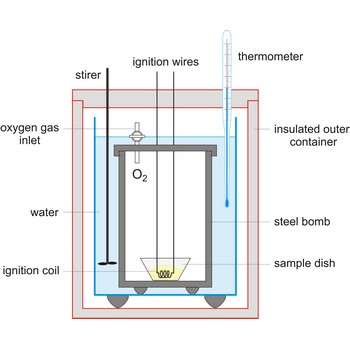
bomb calorimeter → kalorimetrijska bomba
Bomb calorimeter is a type of constant-volume calorimeter used in measuring the heat of combustion of samples which can be burned in oxygen. Four essential parts are required in any bomb calorimeter:
- a bomb or vessel in which the combustible charges can be burned,
- a bucket or container for holding the bomb in a measured quantity of water, together with a stirring mechanism,
- an insulating jacket to protect the bucket from transient thermal stresses during the combustion process, and
- a thermometer or other sensor for measuring temperature changes within the bucket.
dehydrogenation → dehidrogenacija
Dehydrogenation is a chemical reaction in which hydrogen is removed from a compound. Dehydrogenation of organic compounds converts single carbon-carbon bonds into double bonds. It is usually affected by means of a metal catalyst or in biological systems by enzyme dehydrogenases.
borane → borani
Borane is any of the group of compounds of boron and hydrogen (B2H6, B4H10, B5H9, B5H11...), many of which can be prepared by action of acid on magnesium boride (Mg3B2). Boranes are a remarkable group of compounds in that their structures cannot be described using the conventional two-electron covalent bond model.
boron → bor
Boron compounds have been known for thousands of years, but the element was not discovered until 1808 by Sir Humphry Davy (England) and independently by Joseph-Louis Gay-Lussac (France) and L. J. Thenard (France). The origin of the name comes from the Arabic word buraq and the Persian word burah meaning boraks. It is hard, brittle, lustrous black semimetal. Unreactive with oxygen, water, alkalis or acids. Combines with most metals to form borides. Boron is obtained from kernite, a kind of borax (Na2B4O7·10H2O). High purity boron is produced by electrolysis of molten potassium fluroborate and potassium chloride (KCl). Amorphous boron is used in pyrotechnic flares to provide a distinctive green color and in rockets as an igniter.
Citing this page:
Generalic, Eni. "Slana voda." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table