salt water → slana voda
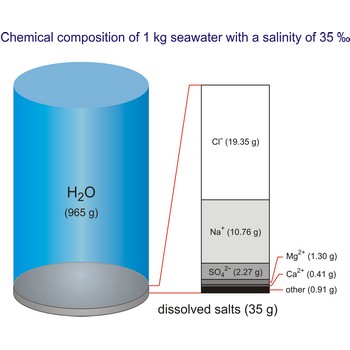
Salt water is the water of the sea and the ocean. This water contains a relatively high percentage of dissolved salt (about 35 g of salt per 1 000 g of sea water.). About 90 % of that salt would be sodium chloride, or ordinary table salt.
The salinity of ocean water varies. It is affected by such factors as melting of ice, inflow of river water, evaporation, rain, etc.
crystal water → kristalna voda
Crystal water is water contained in certain salt crystals. It can be removed by heating. Crystals that contain crystal water are called hydrated and their salts hydrates.
mineral water → mineralna voda
Mineral water is a groundwater that rises to the surface through a natural opening in the earth or rock and contains a relatively high concentration of mineral ions and trace of elements which can be radioactive or thermal.
deionised water → deionizirana voda
Deionised water is water from which ionic salts have been removed by ion-exchange. It is used for many purposes as an alternative to distilled water.
| Type of water | Conductivity / µScm-1 |
|---|---|
| Ultrapure water | 0.05 |
| Distilled water | 0.5 |
| Tap water | 50 |
| Ocean water | 50 000 |
distilled water → destilirana voda
Distilled water is water purified by distillation so as to free it from dissolved salts and other compounds. Distilled water in equilibrium with the carbon dioxide in the air has conductivity of about 0.8×10-6 S cm-1. Repeated distillation in vacuum can bring conductivity down to 0.043×10-6 S cm-1 at 18 °C. The limiting conductivity is due to self ionisation
salt fog chambers → slana komora
Salt fog chambers are designed for corrosive atmosphere testing. The samples being tested are inserted into the chamber and then the salt-containing solution is sprayed as a very fine fog mist over the samples. The temperature within the chamber is maintained constant (usually 35 °C). These test chambers are constructed of non-corrosive materials.
soft water → meka voda
Soft water is any water that does not contain large concentrations of the dissolved minerals calcium or magnesium.
standard mean ocean water → standardna prosječna oceanska voda
Standard mean ocean water (SMOW) is a standard sample of pure water of accurately known isotopic composition which is maintained by the International Atomic Energy Agency. It is used for precise calibration of density and isotopic composition measurements.
waste water → otpadna voda
Waste waters are waters which pour down from housing, public or industrial plants and are polluted with mineral and organic substances and microorganisms.
heavy water → teška voda
Water molecules are composed of two hydrogen atoms and one oxygen atom (H2O). If the hydrogen atoms of a water molecule are replaced by deuterium atoms, the result is heavy water (D2O). Deuterium differs from hydrogen by having one neutron in the nucleus of the atom. There is approx. one part in 5000 D2O in normal water and it can be concentrated by electrolysis. Heavy water has a higher boiling point (101.4 °C) and melts at 3.6 °C. Heavy water is 20/18=1.11 times heavier than ordinary water.
Citing this page:
Generalic, Eni. "Slana voda." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table