Results 1–7 of 7 for sigma veza
sigma bond → sigma veza
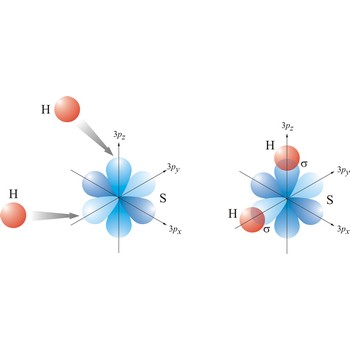
Most single bonds are sigma bonds (σ-bond). In the valence bond theory, a sigma bond is a valence bond that is symmetrical around the imaginary line between the bonded atoms.
potentiometric titration → potenciometrijska titracija
Potentiometric titration is a volumetric method in which the potential between two electrodes is measured (referent and indicator electrode) as a function of the added reagent volume. Types of potentiometric titrations for the determination of analytes in photoprocessing solutions include acid-base, redox, precipitation, and complexometric.
Potentiometric titrations are preferred to manual titrations, since they are more accurate and precise. They are also more easily adapted to automation, where automated titration systems can process larger volumes of samples with minimal analyst involvement.
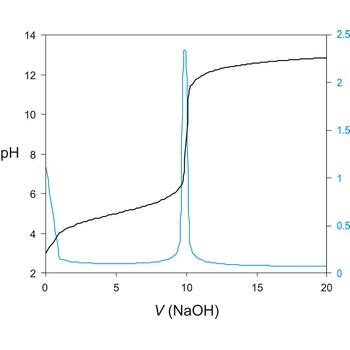
A titration curve has a characteristic sigmoid curve. The part of the curve that has the maximum change marks the equivalence point of the titration. The first derivative, ΔE/ΔV, is the slope of the curve, and the endpoint occurs at the volume, V', where ΔE/ΔV has the maximum value.
standard deviation → standardna devijacija
Standard deviation (σ) is a measure of the dispersion of a set of data from its mean. Standard deviation is a statistical term that measures the amount of variability or dispersion around an average
Suppose there are many measurements of a quantity presumed to be similar, like the size of peas in a pod. If the number of readings for each size were plotted, a bell-shaped curve would probably result, with a few small and large peas and most clustered around the average size. Around two-thirds of all measurements fall in the range spanned by the standard deviation, a measure of the spread.
titration curve → titracijska krivulja
Titration curve is a graphic representation of the amount of a species present vs. volume of solution added during a titration. A titration curve has a characteristic sigmoid curve. The inflection point in the titration curve marks the end-point of the titration. Blue line is the first derivative of the titration curve.
triple bond → trostruka veza
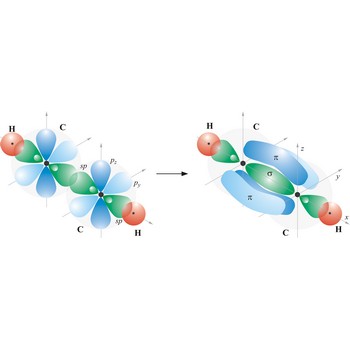
Triple bond. (≡) is a covalent bond that involves 3 bonding pairs. In the valence bond theory, one of the bonds in a triple bond is a sigma bond and the other two are pi bonds. For example, the central bond in acetylene is a triple bond: H-C≡C-H.
volume concentration → volumenska koncentracija
Volume concentration (σ) is equal to volume (VA) of solute and volume (V) of solution proportion. Volume concentration differs from volume fraction because the sum of solution components volume is almost always different than the solution volume.
Lennard-Jones potential → Lennard-Jonesov potencijal
The Lennard-Jones potential (or 12-6 potential) is a mathematically simple model that describes the interaction between two non-bonded and uncharged atoms (known as the van der Waals interaction). It was first proposed in 1924 by British physicist Sir John Edward Lennard-Jones (1894-1954). The Lennard-Jones Potential is given by the following equation
V(r) = 4e[(sigma/r)12-(sigma/r)6)]
where V is the intermolecular potential between the two atoms or molecules, ε is the well depth and a measure of how strongly the two particles attract each other, σ is the distance at which the intermolecular potential between the two particles is zero, r is the distance of separation between centres of both particles.
Citing this page:
Generalic, Eni. "Sigma veza." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table