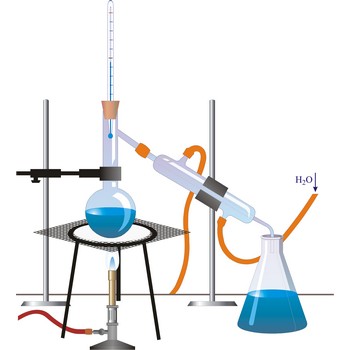
distillation → destilacija
Distillation is a process of boiling a liquid and condensing and collecting the vapour. The liquid collected is the distillate. The usual purpose of distillation is purification or separation of the components of a mixture. This is possible because the composition of the vapour is usually different from that of liquid mixture from which it is obtained. Petrol, kerosene, fuel oil, and lubricating oil are produced from petroleum by distillation.
distilled water → destilirana voda
Distilled water is water purified by distillation so as to free it from dissolved salts and other compounds. Distilled water in equilibrium with the carbon dioxide in the air has conductivity of about 0.8×10-6 S cm-1. Repeated distillation in vacuum can bring conductivity down to 0.043×10-6 S cm-1 at 18 °C. The limiting conductivity is due to self ionisation
heat of hydration → toplina hidratacije
Heat of hydration or enthalpy of hydration of ions corresponds to the heat that is released by hydration of one mole of ions at a constant pressure. The more the ion is hydrated, the more heat is released. Degree of hydration depends on the size and charge of ion. The smaller the ion and the greater its charge, it will be the more hydrated.
heat transfer → prijenos topline
From observations and experiments it has been found that heat energy can be transferred from one position to another through three different modes: conduction, convection and radiation.
dropping mercury electrode → kapajuća živina elektroda
Dropping mercury electrode (DME) is a working electrode arrangement for polarography in which mercury continuously drops from a reservoir through a capillary tube (internal diameter 0.03 - 0.05 mm) into the solution. The optimum interval between drops for most analyses is between 2 and 5 s. The unique advantage to the use of the DME is that the constant renewal of the electrode surface, exposed to the test solution, eliminates the effects of electrode poisoning.
dry cell → suhi članak
Dry cell or Leclanche cell is a primary cell having a zinc anode, a carbon (graphite) cathode surrounded by manganese dioxide, and a paste containing ammonium chloride as electrolyte. The electromotive force (emf) produced by a dry cell is 1.5 V. Dry cell is not reversible and therefore have a limited operating life. It is invented by the French engineer Georges Leclanché (1839.-1882.) in 1866.
dysprosium → disprozij
Dysprosium was discovered by Paul Emile Lecoq de Boisbaudran (France) in 1886. The origin of the name comes from the Greek word dysprositos meaning hard to obtain. It is soft, lustrous, silvery metal. Reacts with oxygen. Reacts rapidly with water; dissolves in acids. Metal ignites and burns readily. Reductant. Dysprosium usually found with erbium, holmium and other rare earths in some minerals such as monazite sand. Dysprosium uses are limited to the experimental and esoteric. Some isotopes of dysprosium are effective absorbers of thermal neutrons and are being considered for use in the control rods in nuclear reactors.
Helmholz free energy → Helmholzova slobodna energija
Helmholz free energy (A) is a thermodynamic function defined by A = U - TS, where U is the internal energy, S the entropy, and T the thermodynamic temperature. For a reversible isothermal process ΔA represents the useful work available.
Citing this page:
Generalic, Eni. "S.t.p.." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table