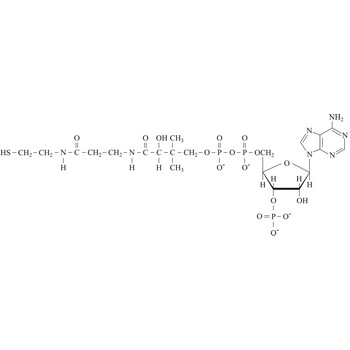
chemical compound formula → formula kemijskog spoja
Chemical elements are represented by their symbols, and chemical compounds are represented by a group of symbols of those elements from which the compound is composed. That group of symbols, which shows which atoms and in which number relation they are present in certain compound is called a chemical compound formula.
In a formula chemical symbols show which element is present in a certain compound, and its index shows how much of that element there is in a certain compound. From sulphuric acid formula H2SO4 we can see that one molecule of sulphuric acid consists of two atoms of hydrogen, one atom of sulphur and four atoms of oxygen.
chromium → krom
Chromium was discovered by Louis-Nicholas Vauquelin (France) in 1797. The origin of the name comes from the Greek word chroma meaning colour. It is very hard, crystalline, steel-grey metal. The pure metal has a blue-white colour. It is hard, brittle and corrosion-resistant at normal temperatures. Hexavalent compounds toxic by skin contact. The most important chromium mineral is chromite [Fe,Mg(CrO4)]. Produced commercially by heating its ore in the presence of silicon or aluminium. Used to make stainless steel. It gives the colour to rubies and emeralds. Iron-nickel-chromium alloys in various percentages yield an incredible variety of the most important metals in modern technology.
cobalt → kobalt
Cobalt was discovered by Georg Brandt (Germany) in 1735. The origin of the name comes from the German word kobald meaning goblin or evil spirit. It is hard, ductile, lustrous bluish-grey metal. Surfaces stable in air. Reacts over time with dilute acids. It has remarkable magnetic properties. Cobalt occurs in compounds with arsenic and sulfur as in cobaltine (CoAsS) and linneite (Co3S4). Pure cobalt is obtained as a by-product of refining nickel, copper and iron. Used in many hard alloys; for magnets, ceramics and special glasses. Radioactive cobalt-60 is used in cancer therapy.
coenzyme a → koenzim a
Coenzyme A (CoA) is an essential metabolic cofactor synthesized from cysteine, pantothenate (vitamin B5), and ATP. CoA plays important roles in many metabolic pathways, including the tricarboxylic acid (TCA) cycle, and the synthesis and oxidation of fatty acids. One of the main functions of CoA is the carrying and transfer of acyl groups. Acylated derivatives (acetyl-CoA) are critical intermediates in many metabolic reactions.
glyceride → glicerid
Glycerides are esters of glycerol (propane-1,2,3-triol) with fatty acids, widely distributed in nature. They are by a long-established custom subdivided into triglycerides, 1,2- or 1,3-diglycerides, and 1- or 2- monoglycerides, according to the number and positions of acyl groups.
Lewis, Gilbert N. → Lewis, Gilbert N.
Gilbert Newton Lewis (1875-1946) is an American chemist whose theory of the electron pair fostered understanding of the covalent bond and extended the concept of acids and bases.
contact procedure → kontaktni postupak
Contact procedure is an industrial procedure used for the production of sulphuric acid, where a dry and clean sulphur dioxide and air go over a catalyst made of vanadium pentoxide at 450 °C by which sulphur trioxide is gained, then we add concentrated sulphuric acid by which we obtain smoking sulphuric acid which is now diluted to sulphuric acid.
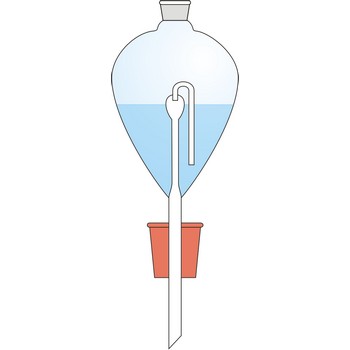
Contat-Gockel’s valve → Contat-Gockelov ventil
Contat-Göckel’s valve is used for maintenance of inert atmosphere in a flask. The valve is filled with a saturated solution of sodium bicarbonate (NaHCO3) so that the end of the tube is covered. Solution inside the valve keeps the flask contents away from the oxygen influence from air. If low pressure is created inside the flask (when the flask is cooled), the solution will penetrate inside it from funnel and in a reaction with acid CO2 is generated which fills up the flask.
Solution from the funnel will keep penetrating until CO2 pressure in the flask is equalised with the outer pressure.
copolymer → kopolimer
Copolymers are also known as heteropolymers. They are made from two (or more) different monomers, which usually undergo a condensation reaction with the elimination of a simple molecule, such as ammonia or water. A typical example is the condensation of 1,6-diaminohexane (hexamethylenediamine) with hexanedioic acid (adipic acid) to form nylon 6,6.
The properties of a polymeric plastic can most easily be modified if it is a copolymer of two or more different monomers, e.g. acrylonitrile-butadiene-styrene copolymer (ABS). Varying the proportions of the component monomers can preselect its properties.
copper → bakar
Copper has been known since ancient times. The origin of the name comes from the Latin word cuprum meaning the island of Cyprus famed for its copper mines. It is malleable, ductile, reddish-brown metal. Resistant to air and water. Exposed surfaces form greenish carbonate film. Pure copper occurs rarely in nature. Usually found in sulfides as in chalcopyrite (CuFeS2), coveline (CuS), chalcosine (Cu2S) or oxides like cuprite (Cu2O). Most often used as an electrical conductor. Also used in the manufacture of water pipes. Its alloys are used in jewellery and for coins.
Citing this page:
Generalic, Eni. "Ribonukleinska kiselina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table