bismuth → bizmut
Bismuth was discovered by Claude Geoffroy (France) in 1753. The origin of the name comes from the German words Weisse Masse meaning white mass; now spelled wismut and bisemutum. It is hard, brittle, steel-grey metal with a pink tint. Stable in oxygen and water. Dissolves in concentrated nitric acid. Bismuth can be found free in nature and in minerals like bismuthine (Bi2S3) and in bismuth ochre (Bi2O3) Main use is in pharmaceuticals and low melting point alloys used as fuses.
borane → borani
Borane is any of the group of compounds of boron and hydrogen (B2H6, B4H10, B5H9, B5H11...), many of which can be prepared by action of acid on magnesium boride (Mg3B2). Boranes are a remarkable group of compounds in that their structures cannot be described using the conventional two-electron covalent bond model.
boron → bor
Boron compounds have been known for thousands of years, but the element was not discovered until 1808 by Sir Humphry Davy (England) and independently by Joseph-Louis Gay-Lussac (France) and L. J. Thenard (France). The origin of the name comes from the Arabic word buraq and the Persian word burah meaning boraks. It is hard, brittle, lustrous black semimetal. Unreactive with oxygen, water, alkalis or acids. Combines with most metals to form borides. Boron is obtained from kernite, a kind of borax (Na2B4O7·10H2O). High purity boron is produced by electrolysis of molten potassium fluroborate and potassium chloride (KCl). Amorphous boron is used in pyrotechnic flares to provide a distinctive green color and in rockets as an igniter.
carboanhidrase → karboanhidraze
Carboanhidrase is an enzyme that catalyzes creation or decomposition of carbonic acid, regulates proportion of carbon dioxide and carbonic acid in the blood plasma.
carbonates → karbonati
Carbonates are compounds composed of metal cation and carbonate anion (CO32-), salts of carbonic acid.
carboxylate group → karboksilna skupina
Carboxylate group (-COOH) is a functional group which is common to all carboxylic acids.
carboxylate ion → karboksilatni ion
Carboxylate ion is gained when carboxylic acid reacts with water.
Bunsen’s cell → Bunsenov članak
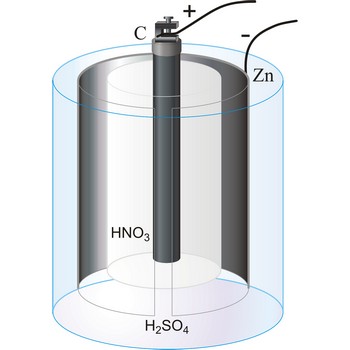
Bunsen’s cell is a primary cell devised by Robert W. Bunsen consisting of a zinc cathode immersed in dilute sulphuric acid and carbon anode immersed in concentrated nitric acid. The electrolytes are separated by a porous pot. The cell gives an e.m.f. of about 1.9 V.
cadmium → kadmij
Cadmium was discovered by Friedrich Strohmeyer (Germany) in 1817. The origin of the name comes from the Latin word cadmia meaning calamine (zinc carbonate, ZnCO3), or from the Greek word kadmeia with the same meaning. It is soft, malleable, blue-white metal. Tarnishes in air, soluble in acids, insoluble in alkalis. Boiling cadmium gives off a weird, yellow-colored vapour that is poisonous. Cadmium can cause a variety of health problems, including kidney failure and high blood pressure. Cadmium is obtained as a by product of zinc refining. The mayor use of cadmium is in electroplating of steel to protect it from corrosion. Also used to make nickel-cadmium batteries. The ability of cadmium to adsorb neutrons has made it of great importance in the design of nuclear reactors. Its compounds are found in paint pigments and a wide variety of intense colours.
carboxylates → karboksilati
Carboxylates is a common name for all salts that carboxylic acids yield by reacting with hydroxides, carbonates, bicarbonates and other alkaline reagents.
Citing this page:
Generalic, Eni. "Ribonukleinska kiselina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table