thallium → talij
Thallium was discovered by Sir William Crookes (England) in 1861. The origin of the name comes from the Greek word thallos meaning green twig or green shoot. It is soft grey metal that looks like lead. Tarnishes in moist air. Reacts in heated moist air and in acids. Compounds highly toxic by inhalation or ingestion. Cumulative effects. Thallium is found in iron pyrites. Also in crookesite, hutchinsonite and lorandite. Most is recovered from the by-products of lead and zinc refining. Its compounds are used in rat and ant poisons. Also for detecting infrared radiation.
thin layer chromatography → tankoslojna kromatografija
Thin layer chromatography. (TLC) is a technique for separating components in a mixture on the basis of their differing polarities. A spot of sample is placed on a flat sheet coated with silica and then carried along by a solvent that soaks the sheet. Different components will move different distances over the surface. TLC is a useful screening technique in clinical chemistry; for example, it can be used to detect the presence of drugs in urine.
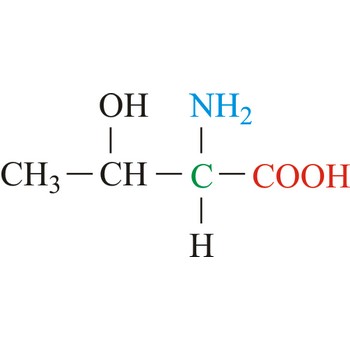
threonine → treonin
Threonine is neutral amino acids with polar side chains. It differs from serine by having a methyl substituent in place of one of the hydrogens on the β carbon. Threonine is a site of phosphorylation and glycosylation which is important for enzyme regulation and cell signaling. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Thr, T
- IUPAC name: 2-amino-3-hydroxybutanoic acid
- Molecular formula: C4H9NO3
- Molecular weight: 119.12 g/mol
thulium → tulij
Thulium was discovered by Per Theodore Cleve (Sweden) in 1879. Named after Thule, an ancient name for Scandinavia. It is soft, malleable, ductile, silvery metal. Tarnishes in air. Reacts with water. Flammable dust. Thulium is found with other rare earths in the minerals gadolinite, euxenite, xenotime and monazite. Radioactive thulium is used to power portable X-ray machines, eliminating the need for electrical equipment.
tin → kositar
Tin has been known since ancient times. The origin of the name comes from the Latin word stannum meaning tin. It is silvery-white, soft, malleable and ductile metal. Exposed surfaces form oxide film. Resists oxygen and water. Dissolves in acids and bases. Organic tin compounds may be highly toxic. Tin is principally found in the ore cassiterite (SnO2) and stannine (Cu2FeSnS4). Used as a coating for steel cans since it is non-toxic and non-corrosive. Also in solder (33 %Sn:67 %Pb), bronze (20 %Sn:80 %Cu) and pewter. Stannous fluoride (SnF2), a compound of tin and fluorine is used in some toothpaste.
titanium → titanij
Titanium was discovered by William Gregor (England) in 1791. Named after the Titans, the sons of the Earth goddess in Greek mythology. It is shiny, dark-grey metal. Powdered form burns in air. Exposed surfaces form oxide coating. It can be highly polished and is relatively immune to tarnishing. Unreactive with alkali and most acids. Titanium usually occurs in the minerals ilmenite (FeTiO3), rutile (TiO2) and iron ores. Pure metal produced by heating TiO2 with C and Cl2 to produce TiCl4 then heated with Mg gas in Ar atmosphere. Since it is strong and resists acids it is used in many alloys. Titanium dioxide (TiO2), a white pigment that covers surfaces very well, is used in paint, rubber, paper and many others.
tryglyceride → triglicerid
Triglyceride is an ester of glycerol and three fatty acids. It is the main constituent of vegetable oil and animal fats. The fatty acids attached to the glycerol can be the same or different. Natural fatty acids found in plants and animals are typically composed only of even numbers of carbon atoms (usually from 16 to 20) due to the way they are bio-synthesized from acetyl CoA.
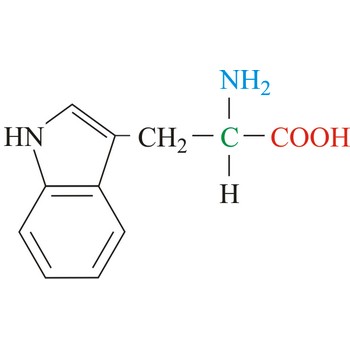
tryptophan → triptofan
Tryptophan is hydrophobic amino acids with aromatic side chain. Tryptophan is large aromatic residue that is normally found buried in the interior of a protein and is important for protein stability. Tryptophan has the largest side chain and is the least common amino acid in proteins. It has spectral properties that make it the best inherent probe for following protein folding and conformational changes associated with biochemical processes. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Trp, W
- IUPAC name: 2-amino-3-(1H-indol-3-yl)propanoic acid
- Molecular formula: C11H12N2O2
- Molecular weight: 204.23 g/mol
tungsten → volfram
Tungsten was discovered by Fausto and Juan Jose de Elhuyar (Spain) in 1783. Named after the tungsten mineral wolframite. It is hard, steel-grey to white metal. Highest melting point of all metals. Resists oxygen, acids and alkalis. Tungsten occurs in the minerals scheelite (CaWO4) and wolframite [(Fe,Mn)WO4]. Made into filaments for vacuum tubes and electric lights. Also as contact points in cars. Tungsten carbide is extremely hard and is used for making cutting tools and abrasives.
tyrosine → tirozin
Tyrosine is hydrophobic amino acids with aromatic side chain. Tyrosine is large aromatic residue that is normally found buried in the interior of a protein and is important for protein stability. Tyrosine has special properties since its hydroxyl side chain may function as a powerful nucleophile in an enzyme active site (when ionized) and is a common site for phosphorylation in cell signaling cascades. Tyrosine absorbs ultraviolet radiation and contributes to the absorbance spectra of proteins. It is not essential (or semi-essential) to the human diet, since it is synthesized in the body from other metabolites.
- Abbreviations: Tyr, Y
- IUPAC name: 2-amino-3-(4-hydroxyphenyl)propanoic acid
- Molecular formula: C9H11NO3
- Molecular weight: 181.19 g/mol
Citing this page:
Generalic, Eni. "Ribonukleinska kiselina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table