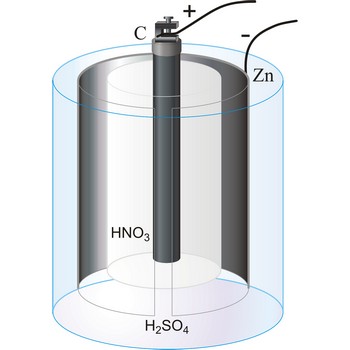
Bunsen’s cell → Bunsenov članak
Bunsen’s cell is a primary cell devised by Robert W. Bunsen consisting of a zinc cathode immersed in dilute sulphuric acid and carbon anode immersed in concentrated nitric acid. The electrolytes are separated by a porous pot. The cell gives an e.m.f. of about 1.9 V.
cadmium → kadmij
Cadmium was discovered by Friedrich Strohmeyer (Germany) in 1817. The origin of the name comes from the Latin word cadmia meaning calamine (zinc carbonate, ZnCO3), or from the Greek word kadmeia with the same meaning. It is soft, malleable, blue-white metal. Tarnishes in air, soluble in acids, insoluble in alkalis. Boiling cadmium gives off a weird, yellow-colored vapour that is poisonous. Cadmium can cause a variety of health problems, including kidney failure and high blood pressure. Cadmium is obtained as a by product of zinc refining. The mayor use of cadmium is in electroplating of steel to protect it from corrosion. Also used to make nickel-cadmium batteries. The ability of cadmium to adsorb neutrons has made it of great importance in the design of nuclear reactors. Its compounds are found in paint pigments and a wide variety of intense colours.
cerium → cerij
Cerium was discovered by Martin Heinrich Klaproth (Germany) and by Jöns Jacob Berzelius (Sweden) in 1803 and Wilhelm von Hisinger (Germany) in 1814. Named after the asteroid Ceres this discovered two years before the element. It is malleable, ductile, iron-grey metal. Tarnishes in air; reacts easily with water. Dissolves in acids; ignites when heated. Metal ignites and burns readily. Strong reductant. Cerium is most abundant rare earth metal. Found in many minerals like monazite sand [Ce(PO4)]. Its oxides are used in the optics and glass-making industries. Its salts are used in the photography and textile industry. Used in high-intensity carbon lamps and as alloying agents in special metals.
conjugated base → konjugirana baza
Conjugated base is a particle which is left after a molecule of acid releases a proton.
conjugated protein → konjugirani protein
Conjugated proteins are proteins which have a prostetic group as a part of their structure which is bonded with one or more amino acids of the same protein.
decomposing → raščinjavanje
Decomposing in analytical chemistry means that a certain substance is converted, by melting it with a suitable melting medium (sodium carbonate, sodium hydroxide, sodium peroxide, ...) in the kind of compound which will afterwards that dissolve in water, acid or base very easily.
detergent → deterdžent
Detergent is a substance added to water to improve its cleaning properties. Although water is a powerful solvent for many compounds, it will not dissolve grease and natural oils. Detergents are compounds that cause such nonpolar substances to go into solution in water. Soap is the original example, owing its action to the presence of ions formed from long-chain fatty acids ion (e.g. stearat ion, CH3(CH2)16COO-).
chemical compound formula → formula kemijskog spoja
Chemical elements are represented by their symbols, and chemical compounds are represented by a group of symbols of those elements from which the compound is composed. That group of symbols, which shows which atoms and in which number relation they are present in certain compound is called a chemical compound formula.
In a formula chemical symbols show which element is present in a certain compound, and its index shows how much of that element there is in a certain compound. From sulphuric acid formula H2SO4 we can see that one molecule of sulphuric acid consists of two atoms of hydrogen, one atom of sulphur and four atoms of oxygen.
chlorine → klor
Chlorine was discovered by Carl William Scheele (Sweden) in 1774. The origin of the name comes from the Greek word chloros meaning pale green. It is greenish-yellow, disagreeable gas with irritating odour. Gas is toxic and severe irritant by contact or inhalation. Never found in free form in nature. Commercial quantities of chlorine are produced by electrolysis of aqueous sodium chloride (NaCl) from seawater or brine from salt mines. Used in water purification, bleaches, acids and many, many other compounds such as chlorofluorocarbons (CFC).
cobalt → kobalt
Cobalt was discovered by Georg Brandt (Germany) in 1735. The origin of the name comes from the German word kobald meaning goblin or evil spirit. It is hard, ductile, lustrous bluish-grey metal. Surfaces stable in air. Reacts over time with dilute acids. It has remarkable magnetic properties. Cobalt occurs in compounds with arsenic and sulfur as in cobaltine (CoAsS) and linneite (Co3S4). Pure cobalt is obtained as a by-product of refining nickel, copper and iron. Used in many hard alloys; for magnets, ceramics and special glasses. Radioactive cobalt-60 is used in cancer therapy.
Citing this page:
Generalic, Eni. "Ribonucleic acids." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. 8 Apr. 2025. <https://glossary.periodni.com>.
Glossary
Periodic Table