condensation → kondenzacija
1. Condensation is a process of changing from a gaseous to a liquid or solid state, usually done by cooling.
2. Condensation, in colloid systems, is a process where smaller particle join in one colloid size particle
3. Condensation, in chemical terms, is a sort of chemical reaction in which small molecules like water, carbon dioxide, or ammonia single out.
critical point → kritična točka
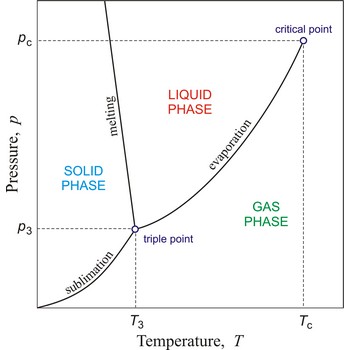
In general, critical point is the point on the phase diagram of a two-phase system at which the two coexisting phases have identical properties and therefore represent a single phase. At the liquid-gas critical point of a pure substance, the distinction between liquid and gas vanishes, and the vapour pressure curve ends. The coordinates of this point are called the critical temperature and critical pressure. Above the critical temperature it is not possible to liquefy the substance.
glacial acetic acid → ledena octena kiselina
Glacial acetic acid (CH3COOH) is the pure compound, as distinguished from the usual water solutions known as acetic acid. It is a colorless liquid or crystalline substance (melting point 16.6 °C) with a pungent, vinegar odor.
hardening → stvrdnjavanje
Hardening is the process of becoming hard or solid by cooling, or drying, or crystallization. For example, the hardening of concrete.
holography → holografija
Holography is a technique for creating a three-dimensional image of an object by recording the interference pattern between a light beam diffracted from the object and a reference beam. The image can be reconstructed from this pattern by a suitable optical system.
degree → stupanj
1. Degree is a unit of temperature on a specified scale. The temperatures of boiling and freezing water are: in the Fahrenheit system 212 and 32 degrees; in the Celsius system 100 and 0 (zero) degrees.
2. Degree is a unit of angular measure. A circle is divided into 360 degrees, represented by the symbol °. Degrees are each divided into 60 minutes. Each minute has 60 seconds. Symbols for degree, minute, and second for plane angle is placed after the numerical value and a no space between the numerical value and the unit symbol (α = 2°3'4").
3. In algebra, the degree of a polynomial is the highest power of the variable in the polynomial. For example, 4x3 + 3x2 + x + 7 have degree 3.
electron configuration → elektronska konfiguracija
The electron configuration shows how many electrons there are in an atom or ion and their distribution along orbitals (see Table of electronic configuration of elements). Structure and all regularity in the periodic system depend upon electronic configuration of atoms of elements. Characteristics of elements mainly depend on electronic configuration of the outer shell. Refilling of the new electronic shell atoms of elements of similar electronic configuration emerge as well as in the previous shell, which adds up to periodicities of characteristics of elements.
irreversible process → ireverzibilni proces
If a system is taken from one state to another but cannot be brought back to the same initial state, then the process is called irreversible. Some examples are free expansion of a gas; dissipation of energy due to friction, or the mixing of two gases or liquids etc.
Citing this page:
Generalic, Eni. "Rhombohedral+crystal+system." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table