antifreeze → antifriz
Antifreeze is a substance added to the liquid (usually water) in the cooling systems of internal-combustion engines to lower its freezing point so that it does not solidify at sub-zero temperatures. The commonest antifreeze is ethane- 1.2-diol (ethylene glycol).
blank determination → slijepa proba
Blank determination is a procedure of determining which follows all steps of analysis but in the absence of a sample. It is used for detection and compensation of systematic analysis mistakes.
Boltzmann equation → Boltzmannova jednadžba
Boltzmann equation is a statistical definition of entropy, given by
where S and k are the entropy and Boltzmann’s constant, respectively, and W is the probability of finding the system in a particular state.
alkynes → alkini
Alkynes (acetylenes) are acyclic branched or unbranched hydrocarbons having one or more triple carbon-carbon bond. In the systematic chemical nomenclature alkyne names end in the suffix -yne. The general formula is CnH(2n+2)-4x were x is the number of triple bonds. Alkynes that have only one triple bond form a homologous series: ethyne (acetylene), CH≡CH, propyne, CH3CH≡CH, etc. Like alkenes, alkynes undergo addition reaction.
allotropic modification → alotropska modifikacija
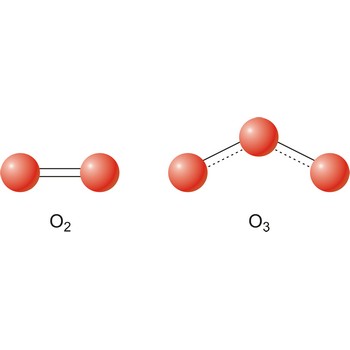
Different substances of the same elementary system are called allotropes or allotropic modifications. In the case of oxygen, there are two allotropic modifications: "normal" dioxygen (O2) and trioxygen (O3) or ozone.
calcination → kalciniranje
Calcination is a process of driving out crystal water from crystal salts by heating.
calibration → kalibracija
Calibration is correcting a measuring instrument by measuring values whose true values are known. Calibration minimises systematic error.
allotropy → alotropija
Allotropy (Gr. allos, other, and tropos, manner) is the phenomenon of an element existing in two or more physical forms in the same physical state. The difference between the forms involves either crystaline structure (white, red and black phosphorus), the number of atoms in the molecule of a gas (diatomic oxygen and triatomic ozone), or the molecular structure of a liquid (liquid helium an helium II).
In some cases, the allotropes are stable over a temperature range, with a definite transition point at which one changes into the other. For instance, tin has two allotropes: white (metallic) tin stable above 13.2 °C and grey (nonmetallic) tin stable below 13.2 °C. This form allotropy is called enantiotropy. Form of allotropy, in which there is no transition temperature at which the two are in equilibrium, is called monotropy.
Allotropy does not apply to the substance existing in different physical states as, for example, when ice melts and changes from solid ice to liquid water.
Allotropy is generally restricted to describing polymorphic behaviour in elements, while polymorphism may refer to any material having multiple crystal structures.
Citing this page:
Generalic, Eni. "Rhombohedral+crystal+system." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table