X-ray crystallography → rendgenska kristalografija
X-ray crystallography is a determination of a three dimensional arrangements of atoms in a crystal by analysis of X-ray diffraction patterns.
allotrope → alotrop
Allotropes are the elements which exist in two or more different forms in the same physical state. Allotropes generally differ in physical properties and may also differ in chemical activity.
Diamond, graphite and fullerenes are three allotropes of the element carbon. Graphite is a soft, black, slippery substance; by contrast, diamond is one of the hardest substances known. The different properties of the allotropes arise from their chemical structures. Diamonds typically crystallize in the cubic crystal system and consist of tetrahedrally bonded carbon atoms. Graphite crystallizes in the hexagonal system. In the fullerenes, the carbon atoms taking the form of a hollow sphere, ellipsoid, or tube.
In some cases, the allotropes are stable over a temperature range, with a definite transition point at which one changes into the other. For instance, tin has two allotropes: white (metallic) tin stable above 13.2 °C and grey (nonmetallic) tin stable below 13.2 °C.
The term allotropes may also be used to refer to the molecular forms of an element. Ozone is a chemically active triatomic allotrope of the element oxygen.
diamond → dijamant
Diamond is the hardest known mineral (with a hardness of 10 on Mohs’ scale). It is an allotropic form of pure carbon that has crystallised in the cubic system, usually as octahedral or cubes, under great pressure. Diamond crystals my be colourless and transparent or yellow, brown or black. They are highly prized as gemstones, but also have extensive uses in industry, mainly for cutting and grinding tools. Diamonds occur in ancient volcanic pipes of kimberlite, or in river deposits that have been derived from weathered kimberlite. Industrial diamonds are being increasingly synthetically produced.
thermodynamic laws → termodinamički zakoni
Thermodynamic laws are the foundation of the science of thermodynamics:
First law: The internal energy of an isolated system is constant; if energy is supplied to the system in the form of heat dq and work dw, then the change in energy dU = dq + dw.
Second law: No process is possible in which the only result is the transfer of heat from a reservoir and its complete conversion to work.
Third law: The entropy of a perfect crystal approaches zero as the thermodynamic temperature approaches zero.
activation energy → energija aktivacije
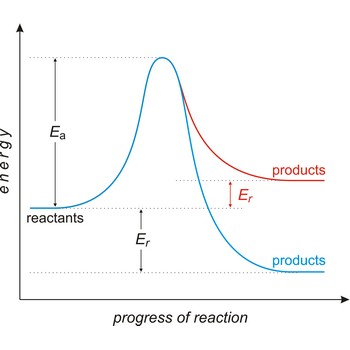
Activation energy (Ea) is the energy that must be added to a system in order for a process to occur, even though the process may already be thermodynamically possible. In chemical kinetics, the activation energy is the height of the potential barrier separating the products and reactants. It determines the temperature dependence on the reaction rate.
adiabatic process → adijabatski proces
Adiabatic process is a thermodynamic process in which no heat enters or leaves the system. In general, an adiabatic change involves a fall or rise in temperature of the system.
alkanes → alkani
Alkanes (paraffins) are acyclic branched or unbranched hydrocarbons having the general formula CnH2n+2, and therefore consisting entirely of hydrogen atoms and saturated carbon atoms. In the systematic chemical nomenclature alkane names end in the suffix -ane. They form a homologous series (the alkane series) methane (CH4), ethane (C2H6), propane (C3H8), butane (C4H10), etc. The lower members of the series are gases; the high-molecular mass alkanes are waxy solid. Generaly the alkanes are fairly unreactive. They form haloalkanes with halogens when irradiated with ultraviolet radiation. Alkanes are present in natural gas and petroleum.
alkenes → alkeni
Alkenes are acyclic branched or unbranched hydrocarbons having one or more double carbon-carbon bonds in their molecules. In the systematic chemical nomenclature, alkene names end in the suffix -ene. The general formula is CnH(2n+2)-2x were x is the number of double bonds. Alkenes that have only one double bond form a homologous series: ethene (ethylene), CH2=CH2, propene, CH3CH2=CH2, etc. Alkenes typically undergo addition reactions to the double bond.
allomerism → alomerija
Allomerism is the appearance of substances with different chemical composition but the same crystalline form.
allomorphism → alomorfija
Allomorphism is the existence of chemical substances with same chemical composition in two or more crystalline forms. See Polymorphism.
Citing this page:
Generalic, Eni. "Rhombohedral+crystal+system." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table