X-ray diffraction → rendgenska difrakcija
The regular array of atoms in a crystal is a three-dimensional diffraction grating for short-wavelength waves such as X-rays. The atoms are arranged in planes with interplanar spacing d. Diffraction maxima occur in the incident direction of the wave, measured from the surface of a plane of atoms, and the wavelength λ of the radiation satisfy Braggs’s law:
X-ray crystallography → rendgenska kristalografija
X-ray crystallography is a determination of a three dimensional arrangements of atoms in a crystal by analysis of X-ray diffraction patterns.
X-ray diffraction pattern → rendgenski difrakcijski uzorci
X-ray diffraction pattern is an interference pattern created by x-rays as they pass through a solid material. Studying X-ray diffraction patterns gives detailed information on the three-dimensional structure of crystals, surfaces, and atoms.
diffraction → difrakcija
Diffraction is the ability of a wave to bend around the edges of obstacles or holes. The effect is most noticeable when the obstacle or hole is comparable to the size of the wavelength
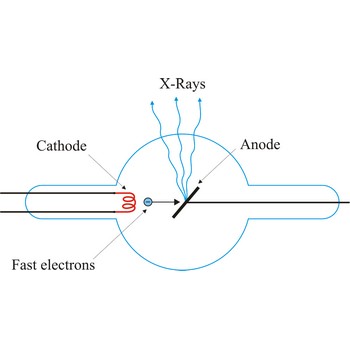
X-ray tube → rendgenska cijev
X-ray tube is a cathode ray tube that focuses energetic streams of electrons on a metal target, causing the metal to emit X-rays. The basic principle of the X-ray tube has not changed significantly since Roentgen's 1895 discovery. Current applied to a metal cathode (about 50 000 V) produces free electrons. The X-rays are produced when the rapidly moving electrons are suddenly stopped as they strike the metal target of the tube.
diffraction grating → difrakcijska rešetka
Diffraction grating is a series of slits used to separate an incident wave into its component wavelengths by directionally separating their diffraction maxima.
Avogadro constant → Avogadrova konstanta
Avogadro constant (NA or L) is the number of elementary entities in one mole of a substance.
It has the value (6.022 045±0.000 031)×1023 mol-1.
Bragg angle → Braggov kut
Bragg angle (Θ) is the angle between an incident X-ray beam and a set of crystal planes for which the secondary radiation displays maximum intensity as a result of constructive interference. British physicist Sir William Henry Bragg and his son Sir William Lawrence Bragg developed a simple relation for scattering angles, now call Bragg’s law.
which relates the angle θ between a crystal plane and the diffracted X-ray beam, the wavelength λ of the x-rays, the crystal plane spacing d, and the diffraction order n (any integer).
The diffraction experiment as presently considered is intended to provide quantitative information on the lattice constant and shape characteristics of the unit cell.
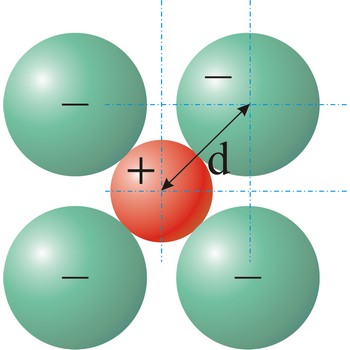
ionic radius → ionski radijus
Ionic radius is the radius of anions and cations in crystalline ionic compounds, as determined by consistently partitioning the center-to-center distance of ions in those compounds. In general, negative ions have larger ionic radii than positive ions.
monochromator → monokromator
Monochromator (from the Greek words mono and chroma meaning single and colour) is an optical device based on dispersion of light by one or more prisms or diffraction gratings into its constituent wavelengths, which are utilized in turn.Citing this page:
Generalic, Eni. "Rendgenska difrakcija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table