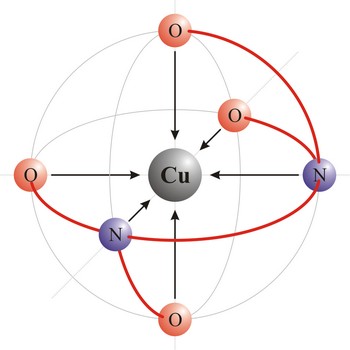
polydentant ligand → polidentantni liganad
Polydentant ligands contain more co-ordination points (can give more electron pairs) and they form complex ringlike structures (celate complexes) by replacing two or more monodentant ligands. That kind of ligand is EDTA which has 6 co-ordinational points and with metals it creates complexes, always in 1:1 ratio.
activated complex → aktivirani kompleks
Activated complex is an intermediate structure formed in the conversion of reactants to products. The activated complex is the structure at the maximum energy point along the reaction path; the activation energy is the difference between the energies of the activated complex and the reactants.
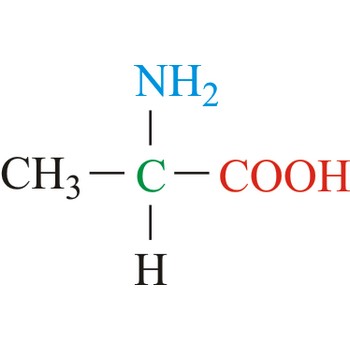
alanine → alanin
Alanine is hydrophobic amino acids with aliphatic side chain. It is the second simplest amino acid, but used the most in proteins. The nonpolar hydrophobic amino acids tend to cluster together within proteins, stabilizing protein structure by means of hydrophobic interactions. Alanine is a nonessential amino acid, meaning it can be manufactured by the human body, and does not need to be obtained directly through the diet.
- Abbreviations: Ala, A
- IUPAC name: 2-aminopropanoic acid
- Molecular formula: C3H7NO2
- Molecular weight: 89.09 g/mol
alkenes → alkeni
Alkenes are acyclic branched or unbranched hydrocarbons having one or more double carbon-carbon bonds in their molecules. In the systematic chemical nomenclature, alkene names end in the suffix -ene. The general formula is CnH(2n+2)-2x were x is the number of double bonds. Alkenes that have only one double bond form a homologous series: ethene (ethylene), CH2=CH2, propene, CH3CH2=CH2, etc. Alkenes typically undergo addition reactions to the double bond.
alkynes → alkini
Alkynes (acetylenes) are acyclic branched or unbranched hydrocarbons having one or more triple carbon-carbon bond. In the systematic chemical nomenclature alkyne names end in the suffix -yne. The general formula is CnH(2n+2)-4x were x is the number of triple bonds. Alkynes that have only one triple bond form a homologous series: ethyne (acetylene), CH≡CH, propyne, CH3CH≡CH, etc. Like alkenes, alkynes undergo addition reaction.
allomerism → alomerija
Allomerism is the appearance of substances with different chemical composition but the same crystalline form.
anion exchange → anionski izmjenjivač
An anionic resin has negative ions built into its structure and therefore exchanges positive ions. In an anion exchange, the side groups are ionised basic groups, such as (-NH2, -NRH, -NR2, -NR3+) to which anions OH- are attached. The exchange reaction is one in which different anions in the solution displace the OH- from the solid.
cation exchange → kationski izmjenjivač
Cation exchange is a cationic resin has positive ions built into its structure and therefore exchanges negative ions. In the cation exchange, the side groups are ionised acidic groups, such as (-SO3H, -COOH, -OH) to which cations H+ are attached. The exchange reaction is one in which different cations in the solution displace the H+ from the solid.
Citing this page:
Generalic, Eni. "Prstenasta struktura." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table