freezing point → ledište
Freezing point is the temperature at which a liquid becomes a solid at normal atmospheric pressure.
See Melting point
glass transition temperature → temperatura staklastog prijelaza
Glass transition temperature (Tg) is the temperature at which an amorphous polymer is transformed, in a reversible way, from a viscous or rubbery condition to a hard and relatively brittle one.
conditional electrode potential → uvjetni elektrodni potencijal
Conditional or formal electrode potential (E°’) is equal to electrode potential (E) when overall concentrations of oxidised and reduced form in all its forms in a solution are equal to one. Conditional electrode potential includes all effects made by reactions that do not take part in the electron exchange, but lead to change of ion power, changes of pH, hydrolysis, complexing, precipitating, etc.
At 298 K (25 °C) and by converting natural (Napierian) logarithms into decimal (common, or Briggian) logarithms, Nernst’s equation for electrode potential can be written as follows:
critical temperature → kritična temperatura
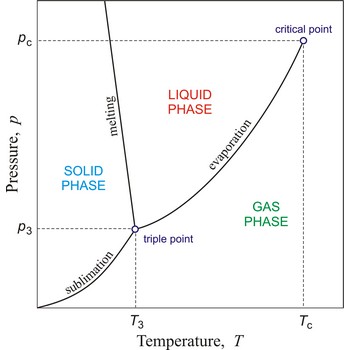
Critical temperature is the temperature of the liquid-vapour critical point, that is, the temperature above which a gas cannot be liquefied by an increase of pressure.
cryogenic fractionation → kriogena frakcinacija
Cryogenic fractionation is a process of separation of gases by cooling them until they enter their liquid state. Large scale gas production companies use this method to produce liquid oxygen, liquid nitrogen etc. Gases have different boiling points (the temperature at which they change from liquid to gas). Oxygen has a boiling point of -183 °C, and nitrogen a boiling point of -195.8 °C. Therefore by cooling the gas mixture to -183 °C, the oxygen can be collected as liquid and the nitrogen remains its gaseous form.
heat of fusion → toplina taljenja
Heat of fusion or enthalpy of fusion is the heat required to convert a substance from the solid to the liquid state with no temperature change (also called latent heat of fusion or melting).
heat of reaction → toplina kemijske reakcije
Heat of reaction or enthalpy of reaction is the heat evolved or absorbed as a result of the complete chemical reaction of molar amounts of the reactants.
heat of sublimation → toplina sublimacije
Heat of sublimation or enthalpy of sublimation is the energy required to convert one mole of a substance from the solid to the gas state (sublimation) without the appearance of the liquid state.
heat of vaporisation → toplina isparavanja
Heat of vaporisation or enthalpy of vaporisation is the heat required to convert a substance from the liquid to the gaseous state with no temperature change (also called latent heat of vaporization).
density → gustoća
In the most common usage, density (ρ) is mass density or mass per unit volume. In Si units it is measured in kg m-3. More commonly, densities are given in kg dm-3.
More generally, it is the amount of some quantity (mass, charge, energy, etc.) divided by a length, area, or volume.
Relative density is the ratio of the density of a substance to the density of some reference substance. For liquids or solids, it is the ratio of the density (usually at 20 °C) to the density of water at 4 °C. This quantity was formerly called specific gravity.
Citing this page:
Generalic, Eni. "Promjena stanja." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table