dynamic equilibrium → dinamička ravnoteža
Dynamic equilibrium is established when two opposing processes are occurring at precisely the same rate, so that there is no apparent change in the system over long periods of time.
chlorinity → klorinitet
Originally chlorinity (symbol Cl) was defined as the weight of chlorine in grams per kilogram of seawater after the bromides and iodides had been replaced by chlorides. To make the definition independent of atomic weights, chlorinity is now defined as 0.3285233 times the weight of silver equivalent to all the halides.
The Mohr-Knudsen titration method served oceanographers for more than 60 years to determine salinity from chlorinity. This modification of the Mohr method uses special volumetric glassware calibrated directly in chlorinity units. The Mohr method uses potassium chromate (K2CrO4) as an indicator in the titration of chloride ions chloride (plus a small amount of bromide and iodide) with a silver nitrate (AgNO3) standard solution.
The other halides present are similarly precipitated.
A problem in the Mohr titration was that silver nitrate is not well suited for a primary standard. The Danish physicist Martin Knudsen (1871-1949) suggested that a standard seawater (Eau de mer Normale or Copenhagen Normal Water) be created and distributed to oceanographic laboratories throughout the world. This water was then used to standardize the silver nitrate solutions. In this way all chlorinity determinations were referred to one and the same standard which gave great internal consistency.
The relationship between chlorinity Cl and salinity S as set forth in Knudsen's tables is
In 1962, however, a better expression for the relationship between total dissolved salts and chlorinity was found to be
extensive property → ekstenzivno svojstvo
Extensive property is a property that changes when the amount of matter in a sample changes. Examples are mass, volume, length, and charge.
cobalt → kobalt
Cobalt was discovered by Georg Brandt (Germany) in 1735. The origin of the name comes from the German word kobald meaning goblin or evil spirit. It is hard, ductile, lustrous bluish-grey metal. Surfaces stable in air. Reacts over time with dilute acids. It has remarkable magnetic properties. Cobalt occurs in compounds with arsenic and sulfur as in cobaltine (CoAsS) and linneite (Co3S4). Pure cobalt is obtained as a by-product of refining nickel, copper and iron. Used in many hard alloys; for magnets, ceramics and special glasses. Radioactive cobalt-60 is used in cancer therapy.
collision theory → teorija sudara
Collision theory is theory that explains how chemical reactions take place and why rates of reaction alter. For a reaction to occur the reactant particles must collide. Only a certain fraction of the total collisions cause chemical change; these are called successful collisions. The successful collisions have sufficient energy (activation energy) at the moment of impact to break the existing bonds and form new bonds, resulting in the products of the reaction. Increasing the concentration of the reactants and raising the temperature bring about more collisions and therefore more successful collisions, increasing the rate of reaction.
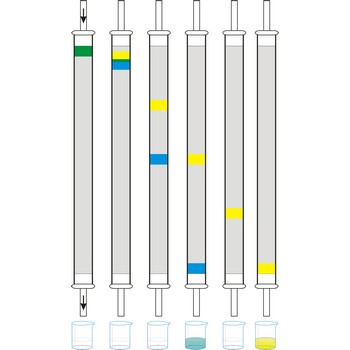
column chromatography → kromatografija u koloni
Column chromatography is generally used as a purification technique: it isolates desired compounds from a mixture. In column chromatography, the stationary phase, a solid adsorbent, is placed in a vertical column. The mobile phase, a liquid, is added to the top and flows down through the column by either gravity or external pressure. The mobile phase can be a gas or a liquid which gives rise to the two basic forms of chromatography, namely, gas chromatography (GC) and liquid chromatography (LC).
condensation → kondenzacija
1. Condensation is a process of changing from a gaseous to a liquid or solid state, usually done by cooling.
2. Condensation, in colloid systems, is a process where smaller particle join in one colloid size particle
3. Condensation, in chemical terms, is a sort of chemical reaction in which small molecules like water, carbon dioxide, or ammonia single out.
fluorescence → fluorescencija
Fluorescence is a luminescence phenomenon in which electron returns to it's ground state almost instantaneously (less than 10-8 second), and in which emission from a luminescent substance ceases when the exciting source is removed. Fluorescence is characterized by radiation emission in all directions.
Citing this page:
Generalic, Eni. "Promjena stanja." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table