protein → bjelančevina
Proteins are natural organic compounds of animal or herbal origin, essential in diet. They are natural polymers developed from a crowd of interconnecting monomers of amino acids, with relative molecular masses amounting up to a few million.
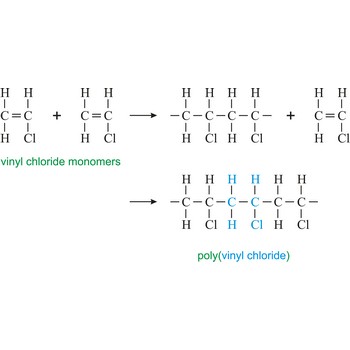
polyvinyl chloride → polivinil klorid
Poly(vinyl chloride) or the PVC is hard and resistant homopolymer produced by the polymerization of the gas vinyl chloride [CH2CHCl]. The pure polymer is hard, brittle and difficult to process, but it becomes flexible when plasticizers are added. After mixing with plasticizers, stabilizers, and pigments, the resin may be fabricated by techniques such as calendering, molding, or extrusion into flexible articles such as raincoats, shower curtains, and packaging films. The resin is not plasticized for use in making rigid products such as water pipe, plumbing fittings, and phonograph records.
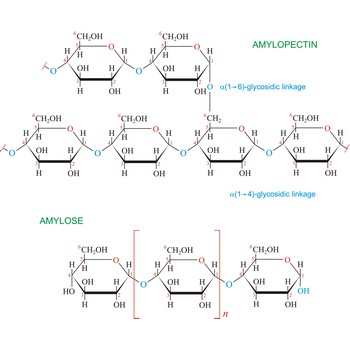
starch → škrob
Starch (C6H10O5)x is a polysaccharide used by plants to stockpile glucose molecules. It is the major component of flour, potatoes, rice, beans, corn, and peas. Starch is a mixture of two different polysaccharides: amylose (about 20 %), which is insoluble in cold water, and amylopectin (about 80 %), which is soluble in cold water. Amylose is composed of unbranched chains of D-glucose units joined by α(1→4)-glycosidic linkages. Unlike amylose, which are linear polymers, amylopectin contains α(1→6)-glycoside branches approximately every 25 glucose units.
Starch digestion begins in the mouth via the action of amylase, a digestive enzyme present in saliva. The process is completed in the small intestine by the pancreatic amylase. The final products of starch digestion, glucose molecules, are absorbed into the intestinal bloodstream and transported to the liver. Like most enzymes, glycosidases are highly selective in their action. They hydrolyze only the α-glycoside links in starch and leave the β-glycoside links in cellulose untouched. Starch is important food stuff and is used in adhesives, and sizes, in laundering, pharmacy and medicine.
terminal → terminalni
Terminal in chemistry means: the end of a polymer molecule and a point at which electron connections can easily be made or broken.
vulcanisation → vulkanizacija
Vulcanisation (vulcanisation of rubber) is a process of combining rubber with sulphur or other substances that causes the polymer chains to crosslink, making them stronger and more elastic.
styrene → stiren
Styrene is an unsaturated hydrocarbon (C6H5OC2H3O) colourless, toxic liquid with a strong aromatic aroma. It is soluble in alcohol, ether, acetone, and carbon disulfide, but dissolves only slightly in water. It is used to make plastics such as polystyrene, ABS, styrene-butadiene rubber styrene-butadiene latex and unsaturated polyesters.
teflon → teflon
Teflon is a DuPont Company trademark for polytetrafluoroethylene (PTFE). It was discovered in 1938 by R. J. Plunkett. Teflon has the lowest coefficient of friction of any solid material known to man. It is also used as a non-stick coating for pans and other cookware. Teflon is very unreactive, and so is often used in containers and pipework for reactive chemicals. Its melting point is 327 °C.
thermosetting plastic → termostabilna plastika
Thermosetting plastics (thermosets) refer to a range of polymer materials that cure, through the addition of energy, to a stronger form. The energy may be in the form of heat (rubber), through a chemical reaction (two part epoxy), or irradiation. Thermoset materials are usually liquid or malleable prior to curing, and designed to be molded into their final form, or used as adhesive.
Thermoset polymer resins transformed into plastics or rubbers by cross-linking into a rigid, 3-D structure. A thermoset material cannot be melted and re-molded after it is cured.
Citing this page:
Generalic, Eni. "Polimer." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table