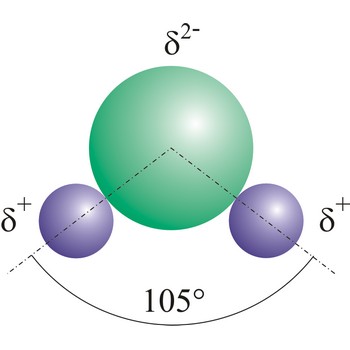
polar molecule → polarna molekula
Polar molecules are molecules at which centres of gravity of positive and negative charge are not in the same point.
dipole molecule → dipolna molekula
Dipole molecules are created when mutual electronic pair at covalent bond is asymmetrical. If different atoms are bonded by a covalent bond, which can have different electron affinity, then the the atom with greater electron affinity will attract the electron pairs more strongly. In this way an asymmetrical distribution of negative charge appears in a molecule, so one part of the molecule becomes relatively negatively (the one closer to the electron pair) and the other becomes relatively positively charged.
polar covalent bond → polarna kovalentna veza
Polar covalent bond is a covalent bond in which the electrons are not equally shared because one atom attracts them more strongly than the other
polyvalent molecule → polivalentna molekula
Polyvalent molecule is a molecule which having multiple binding sites. The antibodies of our immune system are one example.
nonpolar molecule → nepolarna molekula
Nonpolar molecule is a molecule which has no separation of charge, so no positive or negative poles are formed. For example, the Cl2 molecule has no polar bonds (molecule with one type of atom), CH4 is a non-polar molecule (due to its symmetry). Nonpolar molecules do not dissolve in water as they cannot form hydrogen bonds (thus are hydrophobic) but do dissolve in lipids or fats (lipophilic).
polar solvent → polarno otapalo
Polar solvent is a liquid with polar molecules which dissolves polar compounds, because the charges on the endings of the solvent’s molecules attract the elements from ion crystals.
chiral molecule → kiralne molekule
Chiral molecule is a molecule which cannot be superimposed on its mirror image. A common example is an organic molecule containing a carbon atom to which four different atoms or groups are attached. Such molecules exhibit optical activity, i.e., they rotate the plane of a polarised light beam.
molecular formula → molekularna formula
Molecular formula is a formula which represents one molecule of an element or a compound, and the number of atoms in each one of them.
molecular lattice → molekularna rešetka
Molecular lattice is a crystal lattice made molecules bonded together by intermolecular forces.
Citing this page:
Generalic, Eni. "Polarna molekula." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table