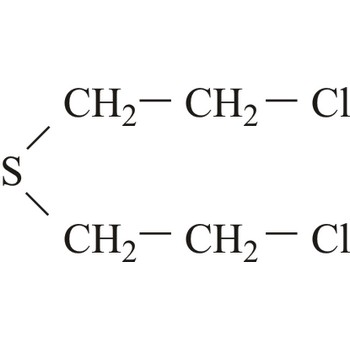gas liquefying → ukapljivanje plinova
In order to achieve transition of a gas into liquid state it is necessary to lower its temperature, or decrease its volume, or increase its pressure. Above the critical temperature it is impossible to liquefy a gas. When liquefying a gas by Linde’s procedure, dampening or Joule-Thomson’s effect is used. First, the compressed air from the compressor is cooled with cooling water, the cooled air expands at a lower pressure in the dampening valve at which it cooled. The cooled air now returns to the compressor, cooling down the expanding air. By repeating this process the air is cooled enough to transit to the liquid state.
universal gas constant → univerzalna plinska konstanta
Universal gas constant R has the value of 8.314 472(15) J K-1 mol-1. It corresponds to the volume work performed by one mole of gas heated by 1 K at standard pressure.
ideal gas law → jednadžba stanja idealnog plina
The generalized ideal gas law is derived from a combination of the laws of Boyle and Charles. Ideal gas law is the equation of state
which defines an ideal gas, where p is pressure, V molar volume, T temperature, and R the molar gas constant (8.314 JK-1mol-1).
mustard agent → plikavac
Mustard agents are usually classified as blistering agents owing to the similarity of the wounds caused by these substances resembling burns and blisters. However, since mustard agents also cause severe damage to the eyes, respiratory system and internal organs, they should preferably be described as blistering and tissue-injuring agents. Normal mustard agent (yperite), 1,1-thio-bis-[2-chloroethane], reacts with a large number of biological molecules. The effect of mustard agent is delayed and the first symptoms do not occur between 2-24 hours after exposure. At room temperature, mustard agent is a liquid with low volatility and is very stable during storage.
adsorption → adsorpcija
Adsorption is a process in which molecules of gas, of dissolved substances in liquids, or of liquids adheres in an extremely thin layer to surfaces of solid bodies with which they are in contact.
aerosol → aerosol
Aerosols are colloidal dispersions of liquid or solid particles in a gas, as in a mist or smoke. The commonly used aerosol sprays contain an inert propellant liquefied under pressure. The pressure of the gas causes the mixture to be released as a fine spray (aerosol) or foam (aerogel) when a valve is opened.
alkanes → alkani
Alkanes (paraffins) are acyclic branched or unbranched hydrocarbons having the general formula CnH2n+2, and therefore consisting entirely of hydrogen atoms and saturated carbon atoms. In the systematic chemical nomenclature alkane names end in the suffix -ane. They form a homologous series (the alkane series) methane (CH4), ethane (C2H6), propane (C3H8), butane (C4H10), etc. The lower members of the series are gases; the high-molecular mass alkanes are waxy solid. Generaly the alkanes are fairly unreactive. They form haloalkanes with halogens when irradiated with ultraviolet radiation. Alkanes are present in natural gas and petroleum.
amount fraction → količinski udio
Amount fraction, xA, (y for gaseous mixtures) is the ratio of the amount of substance (number of moles) of substance A to the total amount of substance in a mixture.
Citing this page:
Generalic, Eni. "Plin." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table