Brownian motion → Braunovo gibanje
Brownian motion is the continuous random movement of small particles suspended in a fluid, which arise from collisions with the fluid molecules. First observed by the British botanist R. Brown (1773-1858) when studying pollen particles. The effect is also visible in particles of smoke suspended in a gas.
cation exchange → kationski izmjenjivač
Cation exchange is a cationic resin has positive ions built into its structure and therefore exchanges negative ions. In the cation exchange, the side groups are ionised acidic groups, such as (-SO3H, -COOH, -OH) to which cations H+ are attached. The exchange reaction is one in which different cations in the solution displace the H+ from the solid.
chemical reaction → kemijska reakcija
Chemical reaction is a change of chemical properties of substances which react with each other. By means of a chemical reaction new substances are created by bond breaking between atoms and molecules of reactants and their reuniting in a new way, thereby creating products. Chemical reactions can be shown by chemical equations.
arginine → arginin
Arginine is an electrically charged amino acids with basic side chains. It is one of the least frequent amino acids. As a group the charged amino acids are important for making proteins soluble. These residues are generally located on the surface of the protein. Arginine is well designed to bind the phosphate anion, and is often found in the active centers of proteins that bind phosphorylated substrates. As a cation, arginine, as well as lysine, plays a role in maintaining the overall charge balance of a protein. Although arginine is considered an essential amino acid (it must be obtained through the diet), this is true only during the juvenile period in humans.
- Abbreviations: Arg, R
- IUPAC name: 2-amino-5-(diaminomethylideneamino)pentanoic acid
- Molecular formula: C6H14N4O2
- Molecular weight: 174.20 g/mol
asparagine → asparagin
Asparagine is neutral amino acids with polar side chains. The polar amino acids are an important class of amino acids since they provide many of the functional groups found in proteins. Asparagine is a common site for attachment of carbohydrates in glycoproteins. In general this is not very reactive residues. Asparagine is amide derivative of aspartic acid. Asparagine is not essential for humans, which means that it can be synthesized from central metabolic pathway intermediates and is not required in the diet.
- Abbreviations: Asn, N
- IUPAC name: 2,4-diamino-4-oxobutanoic acid
- Molecular formula: C4H8N2O3
- Molecular weight: 132.12 g/mol
aspartic acid → asparaginska kiselina
Aspartic acid is an electrically charged amino acids with acidic side chains. As a group the charged amino acids are relatively abundant and are generally located on the surface of the protein. Aspartic acid and glutamic acid play important roles as general acids in enzyme active centers, as well as in maintaining the solubility and ionic character of proteins. Aspartic acid (sometimes referred to as asparate depending on pH) is non-essential in mammals, being produced from oxaloacetate by transamination.
- Abbreviations: Asp, D
- IUPAC name: 2-aminobutanedioic acid
- Molecular formula: C4H7NO4
- Molecular weight: 133.10 g/mol
chlorination → kloriranje
1. Chlorination is an addition or substitution of chlorine in organic compounds.
2. Chlorination is a sterilisation of drinking and swimming pool water or oxidation of undesirable impurities, using chlorine or its compounds.
atmosphere → atmosfera
1. Atmosphere is the column of air which is extending several hundred kilometers above the surface the Earth's surface. The density of this air decreases as you proceed up from the surface. The air in the atmosphere consists of 78 % nitrogen, 21 % oxygen, and 0.9 % argon. The remaining 0.1 % of the atmosphere consists of ozone, water vapor, carbon dioxide, methane, helium, and neon. The atmosphere is divided into different regions. The lowest two layers are the troposphere (the layer closest to the earth) and the stratosphere respectively. These two layers contain more than 99 % of the atmospheric molecules.
2. Standard atmosphere (atm) is an obsolete pressure and stress unit which should be discontinued. It is unit of pressure equal to the air pressure measured at mean sea level.
1 atm = 101 325 Pa
Technical atmosphere (at) is an obsolete MKpS pressure and sttress derived unit.
1 at = 98 066.5 Pa
1 atm = 1.033 227 453 at
atom radius → radijus atoma
Atoms and molecules have no strict boundaries. The volume of a free atom is usually defined as that volume that contains 90 % of electron cloud. The radius of an atom represents half of interatom distance of two identical atoms which are in touch but are not interconnected either by a covalent or an ionic bond, but with a very weak van der Waals’s bond.
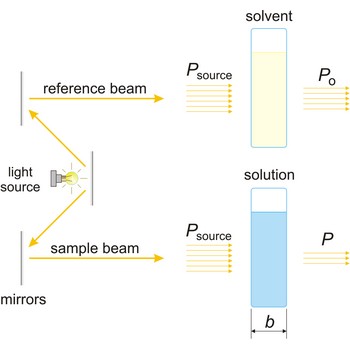
Beer’s law → Beerov zakon
Beer’s law (or Beer-Lambert law) is the functional relationship between the quantity measured in an absorption method (A) and the quantity sought, the analyte concentration (c). As a consequence of interactions between the photons and absorbing particles, the power of the beam is attenuated from Po to P. Beer’s law can be written
where A is the absorbance at a given wavelength of light, ε is the molar absorbtivity or extinction coefficient (L mol-1 cm-1), unique to each molecule and varying with wavelength, b is the length of light path through the sample (cm), and c is the concentration of the compound in solution (mol L-1).
Citing this page:
Generalic, Eni. "Planarna struktura molekule." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table