activated complex → aktivirani kompleks
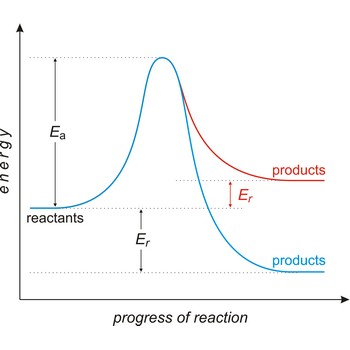
Activated complex is an intermediate structure formed in the conversion of reactants to products. The activated complex is the structure at the maximum energy point along the reaction path; the activation energy is the difference between the energies of the activated complex and the reactants.
activation energy → energija aktivacije
Activation energy (Ea) is the energy that must be added to a system in order for a process to occur, even though the process may already be thermodynamically possible. In chemical kinetics, the activation energy is the height of the potential barrier separating the products and reactants. It determines the temperature dependence on the reaction rate.
allomerism → alomerija
Allomerism is the appearance of substances with different chemical composition but the same crystalline form.
AMU → AMU
AMU or atomic mass unit is a unit of mass used to express relative atomic masses. It is equal to 1/12 of the mass of an atom of the isotope carbon-12 and is equal to 1.66 033×10-27 kg. This unit superseded both the physical and a chemical mass unit based on oxygen-16 and is sometimes called the unified mass unit or the dalton.
anion exchange → anionski izmjenjivač
An anionic resin has negative ions built into its structure and therefore exchanges positive ions. In an anion exchange, the side groups are ionised basic groups, such as (-NH2, -NRH, -NR2, -NR3+) to which anions OH- are attached. The exchange reaction is one in which different anions in the solution displace the OH- from the solid.
anisotropy → anizotropija
Anisotropy is the property of molecules and materials to exhibit variations in physical properties along different molecular axes of the substance.
active site → aktivno mjesto
Active site is a pocket or crevice on an enzyme molecule that fits reactant molecules like a hand in a glove. The active site lowers the activation energy for reaction
activity → aktivitet
Activity (a) is a thermodynamic function used in place of concentration in equilibrium constants for reactions involving nonideal gases and solutions. For the species i activity is defined as
where ai is the activity of the species i, ci is its molar concentration, and fi is a dimensionless quantity called the activity coefficient.
activity coefficient → koeficijent aktiviteta
Activity coefficient (γ or f) is a fractional number which, when multiplied by the molar concentration of a substance in solution, yields the chemical activity. This term gives an idea of how much interaction exists between molecules at higher concentration.
In solutions of very low ionic strength, when m is less than 0.01, the Debye-Hückel limiting law can be used to calculate approximate activity coefficients
where γi = activity coefficient of the species i, zi = charge on the species i and μ = ionic strength of the solution.
Citing this page:
Generalic, Eni. "Planarna struktura molekule." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table