percentage composition of atom in the molecule → postotni sastav atoma u molekuli
Percentage composition of atom in the molecule is a structure of compound presented in the shape of a percentage of its mass, which comes from every element.
polyvalent molecule → polivalentna molekula
Polyvalent molecule is a molecule which having multiple binding sites. The antibodies of our immune system are one example.
structural isomer → strukturni izomer
Structural isomers are compounds that have the same formula, but different constituents. Dimethyl ether (CH3OCH3) and ethanol (CH3CH2OH), for example, are constitutional isomers with general formula C3H6O.
nonpolar molecule → nepolarna molekula
Nonpolar molecule is a molecule which has no separation of charge, so no positive or negative poles are formed. For example, the Cl2 molecule has no polar bonds (molecule with one type of atom), CH4 is a non-polar molecule (due to its symmetry). Nonpolar molecules do not dissolve in water as they cannot form hydrogen bonds (thus are hydrophobic) but do dissolve in lipids or fats (lipophilic).
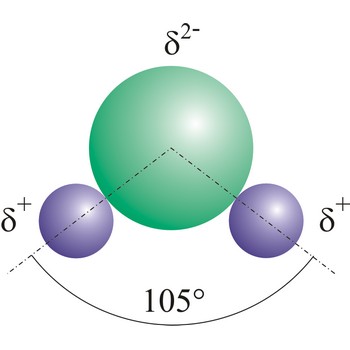
polar molecule → polarna molekula
Polar molecules are molecules at which centres of gravity of positive and negative charge are not in the same point.
relative molecular mass → relativna molekularna masa
Relative molecular mass (Mr) is the ratio of the average mass per molecule or specified entity of a substance to 1/12 of the mass of nuclide 12C. Also called molecular weight. It is equal to the sum of the relative atomic masses of all the atoms that comprise a molecule. For example
Mr(H2SO4) = 2·Ar(H) + Ar(S) + 4·Ar(O)
= 2·1.0079 + 32.066 + 4·15.999
= 2.0158 + 32.066 + 63.996
= 98.078
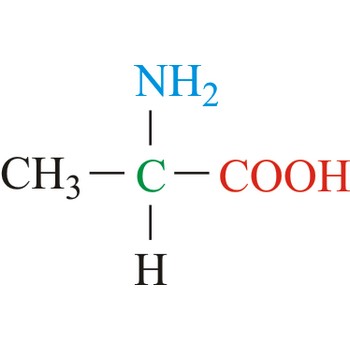
alanine → alanin
Alanine is hydrophobic amino acids with aliphatic side chain. It is the second simplest amino acid, but used the most in proteins. The nonpolar hydrophobic amino acids tend to cluster together within proteins, stabilizing protein structure by means of hydrophobic interactions. Alanine is a nonessential amino acid, meaning it can be manufactured by the human body, and does not need to be obtained directly through the diet.
- Abbreviations: Ala, A
- IUPAC name: 2-aminopropanoic acid
- Molecular formula: C3H7NO2
- Molecular weight: 89.09 g/mol
allotropy → alotropija
Allotropy (Gr. allos, other, and tropos, manner) is the phenomenon of an element existing in two or more physical forms in the same physical state. The difference between the forms involves either crystaline structure (white, red and black phosphorus), the number of atoms in the molecule of a gas (diatomic oxygen and triatomic ozone), or the molecular structure of a liquid (liquid helium an helium II).
In some cases, the allotropes are stable over a temperature range, with a definite transition point at which one changes into the other. For instance, tin has two allotropes: white (metallic) tin stable above 13.2 °C and grey (nonmetallic) tin stable below 13.2 °C. This form allotropy is called enantiotropy. Form of allotropy, in which there is no transition temperature at which the two are in equilibrium, is called monotropy.
Allotropy does not apply to the substance existing in different physical states as, for example, when ice melts and changes from solid ice to liquid water.
Allotropy is generally restricted to describing polymorphic behaviour in elements, while polymorphism may refer to any material having multiple crystal structures.
Citing this page:
Generalic, Eni. "Planarna struktura molekule." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table