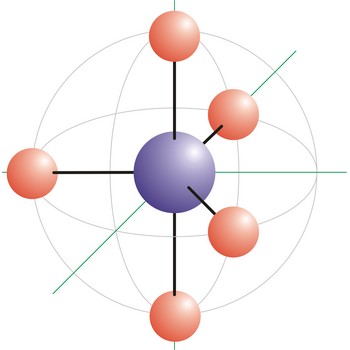
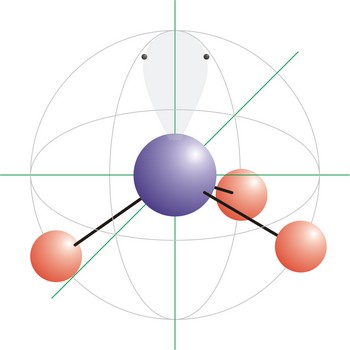
square pyramidal molecular geometry → kvadratna piramidalna geometrija molekule
Square pyramidal is a molecular shape that results when there are five bonds and one lone pair on the central atom in the molecule. Bromine pentafluoride (BrF5) has the geometry of a square pyramid, with fluorine atoms occupying five vertices, one of which is above the plane of the other four. This molecule is made up of six equally spaced sp3d2 (or d2sp3) hybrid orbitals arranged at 90° angles. The shape of the orbitals is octahedral. Because of the high symmetry of the octahedral arrangement, all six positions are equivalent, so it does not matter in which position in the drawing we put the lone pair. The remaining four atoms connected to the central atom give the molecule a square planar shape.
T-shaped molecular geometry → T-oblik geometrije molekule
T-shape is a molecular geometry that results when there are 3 bonds and 2 lone pairs around the central atom in the molecule. The atoms bonded to the central atom lie at the ends of a T with 90° angles between them. Molecules with an trigonal bipyramidal electron pair geometries have sp3d (or dsp3) hybridization at the central atom. ICl3 has a T-shaped molecular geometry.
tetrahedral molecular geometry → tetraedarska geometrija molekule
Tetrahedral is a molecular shape that results when there are four bonds and no lone pairs around the central atom in the molecule. The atoms bonded to the central atom lie at the corners of a tetrahedron with 109.5° angles between them. Molecules with an tetrahedral electron pair geometries have sp3 hybridization at the central atom. The ammonium ion (NH4+) and methane (CH4) have a tetrahedral molecular geometry.
trigonal bipyramidal molecular geometry → trigonska bipiramidalna geometrija molekule
Trigonal bipyramidal (trigonal bipyramidal shape) is a molecular geometry that results when there are five bonds and no lone pairs on the central atom in the molecule. Three of the bonds are arranged along the atom’s equator, with 120° angles between them; the other two are placed at the atom’s axis. Axial bonds are at right angles to the equatorial bonds. Molecules with an trigonal bipyramidal electron pair geometries have sp3d (or dsp3) hybridization at the central atom. The PCl5 molecule has a trigonal bipyramidal molecular geometry.
trigonal pyramidal molecular geometry → trigonska piramidalna geometrija molekule
Trigonal pyramidal is a molecular shape that results when there are three bonds and one lone pair on the central atom in the molecule. Molecules with an tetrahedral electron pair geometries have sp3 hybridization at the central atom. Ammonia (NH3) is a trigonal pyramidal molecule.
structural formula → strukturna formula
Structural formula is a two dimensional representations of the arrangement of the atoms in molecules. Atoms are represented by their element symbols and covalent bonds are represented by lines. The symbol for carbon is often not drawn.
condensed structural formulas → kondenzirane strukturne formule
Condensed structural formulas are shortened and easier layout of structural formulas of organic compounds (butane, CH3(CH2)2CH3).
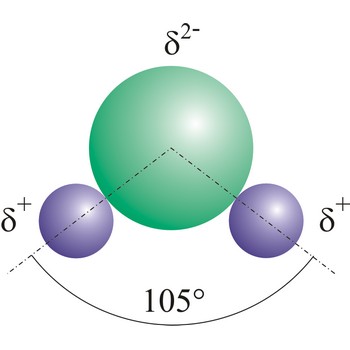
dipole molecule → dipolna molekula
Dipole molecules are created when mutual electronic pair at covalent bond is asymmetrical. If different atoms are bonded by a covalent bond, which can have different electron affinity, then the the atom with greater electron affinity will attract the electron pairs more strongly. In this way an asymmetrical distribution of negative charge appears in a molecule, so one part of the molecule becomes relatively negatively (the one closer to the electron pair) and the other becomes relatively positively charged.
molecular formula → molekularna formula
Molecular formula is a formula which represents one molecule of an element or a compound, and the number of atoms in each one of them.
molecular lattice → molekularna rešetka
Molecular lattice is a crystal lattice made molecules bonded together by intermolecular forces.
Citing this page:
Generalic, Eni. "Planarna struktura molekule." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table