ideal crystal → idealni kristal
Ideal crystal is a single crystal with a perfectly regular lattice that contains no impurities, imperfections, or other defects.
ideal gas → idealni plin
Ideal gas is a gas in which there is complete absence of cohesive forces between the component molecules; the behaviour of such a gas can be predicted accurately by the ideal gas equation through all ranges of temperature and pressure. The concept is theoretical, since no actual gas meets the ideal requirement.
impedance → impedancija
Impedance is the analogue of the resistance or resistivity when applied to alternating current. That is, it is a measure of a material’s inability to carry the electrical current. In many materials the impedance varies as the frequency of the applied electrical potential changes, due to the properties of the conducting liquid or solid. In electrochemistry, the impedance of the electrodes is also frequency dependent.
corrosion → korozija
Corrosion is a harmful and undesirable construction material consumption by the chemical activity of its surroundings. Corrosion concept refers to metal and nonmetal construction materials, but it is usually used for metals, Corrosion of metal, according to the mechanism process, is divided into chemical (corrosion in nonelectrolytes) and electrochemical (corrosion in electrolytes).
Chemical corrosion appears by direct action of molecule of some element or compound on metal, thus directly creating corrosion products.
Electrochemical corrosion of metals occurs in electrolytes, so reduction of metal atom into free cation appears which by secondary processes gives molecules of compound which are considered a corrosion product.
cracking → krekiranje
Cracking is the process whereby heavy molecules of petroleum or crude oil are broken down into hydrocarbons of lower molecular weight (especially in the oil-refining process).
cross-linking → umrežavanje
Cross-linking is an attachment of two chains of polymer molecules by bridges, composed of either an element, a group, or a compound, that join certain carbon atoms of the chains by primary chemical bonds, as indicated in the schematic diagram
Cross-linking occurs in nature in substances made up of polypeptide chains that are joined by the disulfide bonds of the cysteine residue, as in keratins or insulin. Cross-linking can be artificially effected, either adding a chemical substance (cross-linking agent), or by subjecting the polymer to high-energy radiation. Examples are: vulcanisation of rubber with sulphur, cross-linking of polystyrene with divinylbenzene, or cross-linking of polyethylene by means of high-energy radiation.
Cross-linking has the effect of changing a plastic from thermoplastic to thermosetting. Thus, it also increases strength, heat and electrical resistance, and especially resistance to solvents and other chemicals.
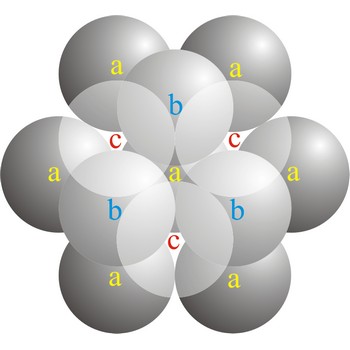
cubic close-packed structure → kubična gusta slagalina
In a cubic close-packed (ccp) arrangement of atoms, the unit cell consists of four layers of atoms. The top and bottom layers (a) contain six atoms at the corners of a hexagon and one atom at the center of each hexagon. The atoms in the second layer (b) fit into depressions in the first layer. The atoms in the third layer (c) occupy a different set of depressions than those in the first. The cubic close packed structure can be made by piling layers in the a-b-c-a-b-c-a-b-c... sequence.
intermediate → intermedijer
Intermediate is a molecular or ionic species that is formed (directly or indirectly) from the reactants and reacts further (directly or indirectly) to form the products of the reaction. It does not accumulate during the course of the reaction.
ionophore → ionofore
Ionophore is a relatively small hydrophobic molecule that facilitates the transport of ions across lipid membranes. Most ionophores are produced by microorganisms. There are two types of ionophores: channel formers, which combine to form a channel in the membrane through which ions can flow; and mobile ion carriers, which transport ions across a membrane by forming a complex with the ion.
Citing this page:
Generalic, Eni. "Planarna struktura molekule." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table