peptide bond → peptidna veza
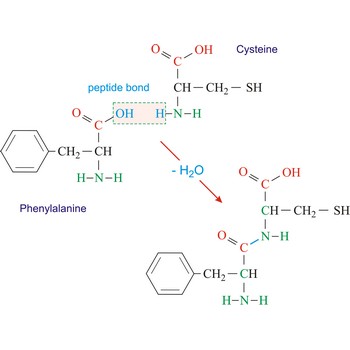
Peptide bond emerges when two amino acid join in a way that the carbon atom from one connects with the nitrogen atom from the other (creating a C-N bond).
sigma bond → sigma veza
Most single bonds are sigma bonds (σ-bond). In the valence bond theory, a sigma bond is a valence bond that is symmetrical around the imaginary line between the bonded atoms.
triple bond → trostruka veza
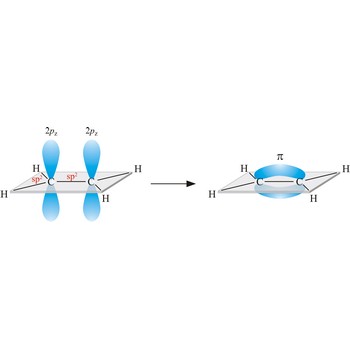
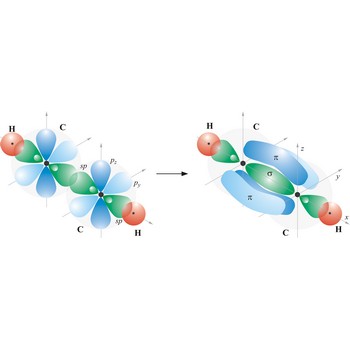
Triple bond. (≡) is a covalent bond that involves 3 bonding pairs. In the valence bond theory, one of the bonds in a triple bond is a sigma bond and the other two are pi bonds. For example, the central bond in acetylene is a triple bond: H-C≡C-H.
valence bond theory → teorija valentne veze
Valence bond theory is a theory that explains the shapes of molecules in terms of overlaps between half-filled atomic orbitals, or half filled hybridised orbitals.
addition reactions → reakcije adicije
Addition reactions are normally occur with unsaturated compounds and involve the addition of one molecule (called the reactant) across the unsaturated bond (i.e. the double bond or the triple bond) of another molecule (called the substrate) to give a single product, formed by the combination of both reacting molecules.
For example, bromine adds across the double bond of ethene in an addition reaction to form dibromoethane.
Citing this page:
Generalic, Eni. "Pi veza." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table