antimony → antimon
Antimony has been known since ancient times. The origin of the name comes from the Latin word stibium meaning mineral stibnite. It is hard, brittle, silvery-white semimetal. Stable in dry air. Toxic by ingestion or inhalation. Antimony is found in stibnite (Sb2S3) and in valentinite (Sb2O3). It is alloyed with other metals to increase their hardness. Also in the manufacture of a few special types of semiconductor devices. Also in plastics and chemicals. A few kinds of over-the-counter cold and flu remedies use antimony compounds.
arsenic → arsen
Arsenic was discovered by Albertus Magnus (Germany) in 1250. The origin of the name comes from the Greek word arsenikon meaning yellow orpiment. It is steel-grey, brittle semi-metal. Resists water, acids and alkalis. Tarnishes in air, burns in oxygen. Highly toxic by inhalation or ingestion. Arsenic is found in mispickel (arsenopyrite). Many of its compounds are deadly poison and used as weed killer and rat poison. Used in semiconductors. Some compounds, called arsenides, are used in the manufacture of paints, wallpapers and ceramics.
atmosphere → atmosfera
1. Atmosphere is the column of air which is extending several hundred kilometers above the surface the Earth's surface. The density of this air decreases as you proceed up from the surface. The air in the atmosphere consists of 78 % nitrogen, 21 % oxygen, and 0.9 % argon. The remaining 0.1 % of the atmosphere consists of ozone, water vapor, carbon dioxide, methane, helium, and neon. The atmosphere is divided into different regions. The lowest two layers are the troposphere (the layer closest to the earth) and the stratosphere respectively. These two layers contain more than 99 % of the atmospheric molecules.
2. Standard atmosphere (atm) is an obsolete pressure and stress unit which should be discontinued. It is unit of pressure equal to the air pressure measured at mean sea level.
1 atm = 101 325 Pa
Technical atmosphere (at) is an obsolete MKpS pressure and sttress derived unit.
1 at = 98 066.5 Pa
1 atm = 1.033 227 453 at
coal → ugljen
Coal is a black or brownish-black, combustible sedimentary rock, with 30 % (lignite) to 98 % (anthracite) carbon by weight, mixed with various amounts of water and small amounts of sulfur and nitrogen compounds. It is formed from plant matter that decayed in swamps and bogs that has been compressed and altered by geological processes over millions of years. Coal is primarily used as a fuel.
colloid silver → koloidno srebro
Colloid silver is a bright blue-green powder which dissolved in water gives colloid solution of red colour.
barium → barij
Barium was discovered by Sir Humphry Davy (England) in 1808. The origin of the name comes from the Greek word barys meaning heavy. It is soft, slightly malleable, silvery-white metal. Attacked by air and water. Soluble compounds toxic by ingestion. Barium is found in barytine (BaSO4) and witherite (BaCO3), never found in pure form due to its reactivity. Must be stored under kerosene to remain pure. Barite, or barium sulfate (BaSO4), when ground is used as a filter for rubber, plastics and resins. It is insoluble in water and so is used in X-rays of the digestive system. Barium nitrate, Ba(NO3)2, burns brilliant green and is used in fireworks.
bismuth → bizmut
Bismuth was discovered by Claude Geoffroy (France) in 1753. The origin of the name comes from the German words Weisse Masse meaning white mass; now spelled wismut and bisemutum. It is hard, brittle, steel-grey metal with a pink tint. Stable in oxygen and water. Dissolves in concentrated nitric acid. Bismuth can be found free in nature and in minerals like bismuthine (Bi2S3) and in bismuth ochre (Bi2O3) Main use is in pharmaceuticals and low melting point alloys used as fuses.
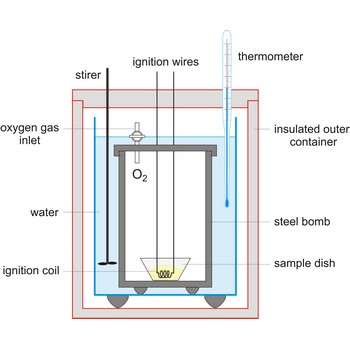
bomb calorimeter → kalorimetrijska bomba
Bomb calorimeter is a type of constant-volume calorimeter used in measuring the heat of combustion of samples which can be burned in oxygen. Four essential parts are required in any bomb calorimeter:
- a bomb or vessel in which the combustible charges can be burned,
- a bucket or container for holding the bomb in a measured quantity of water, together with a stirring mechanism,
- an insulating jacket to protect the bucket from transient thermal stresses during the combustion process, and
- a thermometer or other sensor for measuring temperature changes within the bucket.
boron → bor
Boron compounds have been known for thousands of years, but the element was not discovered until 1808 by Sir Humphry Davy (England) and independently by Joseph-Louis Gay-Lussac (France) and L. J. Thenard (France). The origin of the name comes from the Arabic word buraq and the Persian word burah meaning boraks. It is hard, brittle, lustrous black semimetal. Unreactive with oxygen, water, alkalis or acids. Combines with most metals to form borides. Boron is obtained from kernite, a kind of borax (Na2B4O7·10H2O). High purity boron is produced by electrolysis of molten potassium fluroborate and potassium chloride (KCl). Amorphous boron is used in pyrotechnic flares to provide a distinctive green color and in rockets as an igniter.
condensational polymerisation → kondenzacijska polimerizacija
Condensational polymerisation is a reaction of polymerisation in which monomers together create a polymer by losing small molecules like water.
Citing this page:
Generalic, Eni. "Permanent hardness in water." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table