gadolinium → gadolinij
Gadolinium was discovered by Jean de Marignac (France) in 1880. Named after the mineral gadolinite, named for J. Gadolin, a Finnish chemist and mineralogist. It is soft, ductile, silvery-white metal. Reacts slowly with water and oxygen. Dissolves in acids. Metal ignites and burns readily. Gadolinium is found with other rare earths in gadolinite and monazite sand. Used in steel alloying agents and the manufacture of electronic components.
gallium → galij
Gallium was discovered by Lecoq de Boisbaudran (France) in 1875. The origin of the name comes from the Latin word Gallia meaning France. It is soft, blue-white metal. Stable in air and water. Reacts violently with chlorine and bromine. Gallium is found throughout the crust in minerals like bauxite, germanite and coal. Used in semiconductor production. It us used in making LED’s (light-emitting diodes) and GaAs laser diodes.
gas liquefying → ukapljivanje plinova
In order to achieve transition of a gas into liquid state it is necessary to lower its temperature, or decrease its volume, or increase its pressure. Above the critical temperature it is impossible to liquefy a gas. When liquefying a gas by Linde’s procedure, dampening or Joule-Thomson’s effect is used. First, the compressed air from the compressor is cooled with cooling water, the cooled air expands at a lower pressure in the dampening valve at which it cooled. The cooled air now returns to the compressor, cooling down the expanding air. By repeating this process the air is cooled enough to transit to the liquid state.
germanium → germanij
Germanium was discovered by Clemens Winkler (Germany) in 1886. The origin of the name comes from the Latin word Germania meaning Germany. It is greyish-white semi-metal. Unaffected by alkalis and most (except nitric) acids. Stable in air and water. Germanium is obtained from refining copper, zinc and lead. Widely used in semiconductors. It is a good semiconductor when combined with tiny amounts of phosphorus, arsenic, gallium and antimony.
global hectare → globalni hektar
Global hectares (gha) are hectares with world-average productivity for all productive land and water areas in a given year. Because different land types have different productivity, a global hectare of, for example, cropland, would occupy a smaller physical area than the much less biologically productive pasture land, as more pasture would be needed to provide the same biocapacity as one hectare of cropland ("ordinary" hectare is an area equal to a square that is 100 meters on each side, so a hectare has 10 000 m2). Global hectare is unit for measuring our demands on the Earth (ecological footprint) and the ability of the Earth to supply our demands (biocapacity).
glucose → glukoza
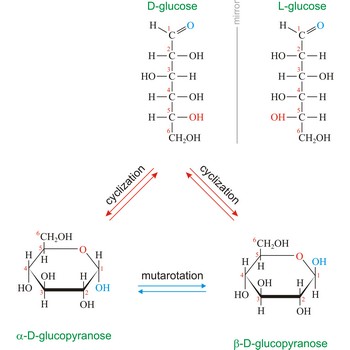
Glucose (grape sugar, blood sugar), C6H12O6, is an aldohexose (a monosaccharide sugar having six carbon atoms and an aldehyde group). An older common name for glucose is dextrose, after its dextrorotatory property of rotating plane polarized light to the right. Glucose in free (in sweet fruits and honey) or combined form (sucrose, starch, cellulose, glycogen) is is probably the most abundant organic compound in nature. During the photosynthesis process, plants use energy from the sun, water from the soil and carbon dioxide gas from the air to make glucose. In cellular respiration, glucose is ultimately broken down to yield carbon dioxide and water, and the energy from this process is stored as ATP molecules (36 molecules of ATP across all processes).
Naturally occurring glucose is D isomers (OH group on the stereogenic carbon farthest from the aldehyde group, C-5, is to the right in the Fischer projection). Although often displayed as an open chain structure, glucose and most common sugars exist as ring structures. In the α form, the hydroxyl group attached to C-1 and the CH2OH attached to C-5 are located on opposite sides of the ring. β-glucose has these two groups on the same side of the ring. The full names for these two anomers of glucose are α-D-glucopyranose and β-D-glucopyranose.
gold → zlato
Gold has been known since ancient times. The origin of the name comes from the Latin word aurum meaning gold. It is soft, malleable, bright yellow metal. Unaffected by air, water, alkalis and most acids. Gold is found in veins in the crust, with copper ore and native. Used in electronics, jewellery and coins. It is a good reflector of infrared radiation, so a thin film of gold is applied to the glass of skyscrapers to reduce internal heating from sunlight.
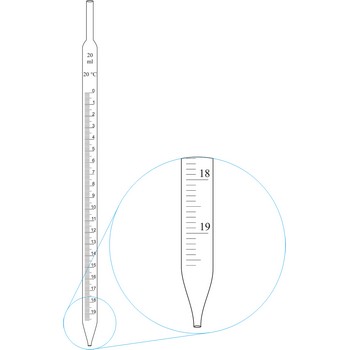
graduated pipette → graduirana pipeta
Graduated pipettes (Mohr pipette) have a scale divided into units of one and of 1/10th of a millilitre. Because of their wide necks it is less accurate than the volumetric pipette. They are used when taking volume of solutions in which accuracy does not have to be very high. By sucking in (with mouth, propipette or a water pump) the liquid is pulled in a little bit above the mark and the opening of the pipet is closed with a forefingertip. Outer wall of pipet is wiped and, with a slight forefinger loosening, the liquid is released until it reaches the mark 0. A pipette is emptied out by lifting the forefinger off and letting the liquid flow out of the pipette freely.
Haber process → Haberov proces
Haber process is an industrial process for producing ammonia by reaction of nitrogen with hydrogen:
The reaction is reversible and exothermic, so that a high yield of ammonia is favoured by low temperature. However, the rate of reaction would be too slow for equilibrium to be reached at normal temperatures, so an optimum temperature of about 450 °C is used, with a catalyst of iron containing potassium aluminium oxide promoters. The higher the pressure the greater the yield, although there are technical difficulties in using very high pressures. A pressure of about 250 atmospheres is commonly employed. The removal of ammonia from the batch as soon as it is formed ensures that an equilibrium favouring product formation is maintained. The nitrogen is obtained from air. Formerly, the hydrogen was from water gas and the water-gas shift reaction (the Bosch process) but now the raw material (called synthesis gas) is obtained by steam reforming natural gas.
The process is of immense importance for the fixation of nitrogen for fertilisers and explosives. It was developed in 1908 by German chemist Fritz Haber (1868-1934) and was developed for industrial use by Carl Bosch (1874-1940), hence the alternative name Haber-Bosch process.
half-cell → polučlanak
Half-cell is a part of galvanic cell in which oxidations or reduction of an element in contact with water or water solution one of its compounds.
Citing this page:
Generalic, Eni. "Permanent hardness in water." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table