diamond → dijamant
Diamond is the hardest known mineral (with a hardness of 10 on Mohs’ scale). It is an allotropic form of pure carbon that has crystallised in the cubic system, usually as octahedral or cubes, under great pressure. Diamond crystals my be colourless and transparent or yellow, brown or black. They are highly prized as gemstones, but also have extensive uses in industry, mainly for cutting and grinding tools. Diamonds occur in ancient volcanic pipes of kimberlite, or in river deposits that have been derived from weathered kimberlite. Industrial diamonds are being increasingly synthetically produced.
diatomaceous earth → dijatomejska zemlja
Diatomaceous earth is a naturally occurring siliceous sedimentary mineral compound from microscopic skeletal remains (frustules) of diatoms, unicellular aquatic plants of microscopic size. Their fossilized remains are called diatomite and contains approximately 3000 diatom frustules per cubic millimetre.
Diatomite is relatively inert and has a high absorptive capacity, large surface area, and low bulk density. It consists of approximately 90 % silica, and the remainder consists of compounds such as aluminum and iron oxides. The fine pores in the diatom frustules make diatomite an excellent filtering material for waters, beverages, oils, chemicals, as well as many other products.
dielectric constant → dielektrična konstanta
Dielectric constant or permittivity (ε) is an index of the ability of a substance to attenuate the transmission of an electrostatic force from one charged body to another. The lower the value, the greater the attenuation. The standard measurement apparatus utilises a vacuum whose dielectric constant is 1. In reference to this, various materials interposed between the charged terminal have the following value at 20 °C:
| vacuum | 1 |
| air | 1.00058 |
| glass | 3 |
| benzene | 2.3 |
| acetic acid | 6.2 |
| ammonia | 15.5 |
| ethanol | 25 |
| glycerol | 56 |
| water | 81 |
The exceptionally high value for water accounts for its unique behaviour as a solvent and in electrolytic solutions. Dielectric constant values decrease as the temperature rises.
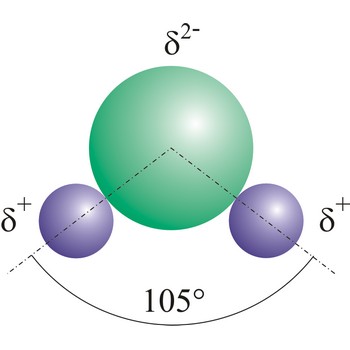
dipole molecule → dipolna molekula
Dipole molecules are created when mutual electronic pair at covalent bond is asymmetrical. If different atoms are bonded by a covalent bond, which can have different electron affinity, then the the atom with greater electron affinity will attract the electron pairs more strongly. In this way an asymmetrical distribution of negative charge appears in a molecule, so one part of the molecule becomes relatively negatively (the one closer to the electron pair) and the other becomes relatively positively charged.
dissociation → disocijacija
Dissociation is the process by which a chemical combination breaks up into simpler constituents as a result of either added energy (dissociated by heat), or the effect of a solvent on a dissolved polar compound (electrolytic dissociation). It may occur in the gaseous, solid, or liquid state, or in a solution.
An example of dissociation is the reversible reaction of hydrogen iodide at high temperatures
The term dissociation is also applied to ionisation reactions of acids and bases in water. For example
which is often regarded as a straightforward dissociation into ions
strong electrolyte → jaki elektrolit
Strong electrolytes are those electrolytes which in water solutions completely dissociate into their ions. They conduct electric current very well.
thermal pollution → toplinsko zagađenje
Thermal pollution is the increase in temperature of natural waters resulting from the discharge to these waters of hot effluents from industrial and power plants. The higher temperatures reduce the concentration of dissolved oxygen.
Citing this page:
Generalic, Eni. "Permanent hardness in water." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table