nerve poison → živčani bojni otrov
Nerve poison (nerve gas, agents) have had an entirely dominant role since the Second World War. Nerve poisons acquired their name because they affect the transmission of nerve impulses in the nervous system. All nerve poisons belong chemically to the group of organo-phosphorus compounds. They are stable and easily dispersed, highly toxic and have rapid effects both when absorbed through the skin and via respiration. Nerve poisons can be manufactured by means of fairly simple chemical techniques. The raw materials are inexpensive and generally readily available.
The most important nerve agents included in modern chemical weapons arsenals are:
| Tabun | (o-ethyl dimethylamidophosphorylcyanide) |
| Sarin | (isopropyl methylphosphonofluoridate) |
| Soman | (pinacolyl methylphosphonofluoridate) |
| GF | (cyclohexyl methylphosphonofluoridate) |
| VX | (o-ethyl S-diisopropylaminomethyl methylphosphonothiolate) |
Nerve poisons are colorless, odorless, tasteless liquids of low volatility. Antidotes are atropine sulfate and pralidoxime iodide.
Newman’s projection → Newmanova projekcija
Newman’s projection is an image which we get when we observe a model of ethane molecule in C-C bond direction.
niobium → niobij
Niobium was discovered by Charles Hatchett (England) in 1801. The origin of the name comes from the Greek word Niobe meaning daughter of Tantalus in Greek mythology (tantalum is closely related to niobium in the periodic table). It is shiny white, soft, ductile metal. Exposed surfaces form oxide film. Niobium occurs in a mineral columbite. It is used in stainless steel alloys for nuclear reactors, jets and missiles. Used as an alloy with iron and nickel. It can be used in nuclear reactors and is known to be superconductive when alloyed with tin, aluminium or zirconium.
noble gas → plemeniti plin
Noble gas refers to any element of the group of six elements in group 18 of the periodic table. They are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). Unlike most elements, the noble gases are monoatomic. The atoms have stable configurations of electrons. Therefore, under normal conditions they do not form compounds with other elements.
They were generally called inert gases until about 1962 when xenon tetrafluoride, XeF4, was produced in the laboratory. This was the first report of a stable compound of a noble gas with another single element.
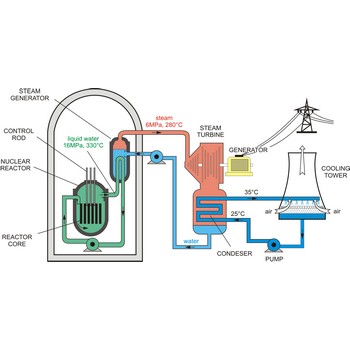
nuclear reactor → nuklearni reaktor
Nuclear reactor is an assembly of fissionable material (uranium-235 or plutonium-239) designed to produce a sustained and controllable chain reaction for the generation of electric power.
The essential components of a nuclear reactor are:
- The core, metal rods containing enough fissionable material to maintain a chain reaction at the necessary power level (as much as 50 t of uranium may be required).
- A source of neutrons to initiate the reaction (such as a mixture of polonium and beryllium)
- A moderator to reduce the energy of fast neutrons for more efficient fission (material such as graphite, beryllium, heavy water, and light water are used)
- A coolant to remove the fission-generated heat (water, sodium, helium, and nitrogen may be used)
- A control system such as rods of boron or cadmium that have high capture cross sections (to absorb neutrons)
- Adequate shielding, remote-control equipment, and appropriate instrumentation are essential for personnel safety and efficient operation.
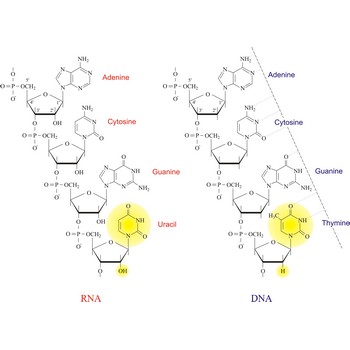
nucleic acid → nukleinska kiselina
Nucleic acids are a complex, high-molecular-weight biochemical macromolecules composed of nucleotide chains that convey genetic information. The most common nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Each nucleic acid chain is composed of subunits called nucleotides, each containing a sugar, a phosphate group, and nitrogenous base. DNA was first discovered in 1869 by the Swiss biochemist Friedrich Miescher (1844-1895).
Both DNA and RNA contain the two major purine bases adenine (A) and guanine (G) and one of the major pyrimidines, cytosine (C). Of the other two pyrimidines, thymine (T) is found in DNA and uracil (U) is found in RNA. There are two major pentoses in nucleic acids:2'-deoxy-D-ribose in DNA and D-ribose in RNA.
Nucleotides are linked together in both DNA and RNA in a polymeric fashion via covalent bonds. These bonds exist through phosphate-group bridges in which the 5' hydroxyl group of one nucleotide unit is joined to the 3' hydroxyl group of the next nucleotide. RNA is usually a single-stranded molecule, whereas DNA is usually double-stranded.
nucleotide → nukleotid
Nucleotides are the components that made up nucleic acids. They have three major components: the first component is a nitrogenous base, which is derivative of one of two parent compounds, pyrimidine or purine; the second is a pentose, or five carbon sugar group; the third is a unit of phosphate. Each group of three nucleotides in a gene is known as a codon. Whenever the phosphate group is not present, a nucleotide becomes a nucleoside.
octahedral molecular geometry → oktaedarska geometrija molekule
Octahedral molecular geometry (square bipyramidal shape) describes the shape of compounds where six atoms or ligands are symmetrically arranged around a central atom. The sulfur hexafluoride (SF6), with six bonding pairs, is predicted and found to be a regular octahedron. Four of the attachments are positioned in a square plane with 90° bond angles. The remaining two attachments are positioned perpendicular (90°) to the square plane at opposite ends of the central atom. Molecules with an octahedral electron pair geometries have sp3d2 (or d2sp3) hybridization at the central atom.
octahedron → oktaedar
Octahedron is a three-dimensional geometric figure having eight triangular sides.
octet rule → pravilo okteta
Octet rule states that the chemical properties of the elements repeat on a regular basis with increasing atomic mass, and that the chemical properties of each eight element are similar. Since the inert gases, with the exception of helium have eight electrons in their outer shells, this stable electronic configuration is called the octet rule. In chemical reactions atoms of elements tend to react in such a way as to achieve the electronic configuration of the inert gas nearest to them in the periodic table. There are a number of exceptions to the octet rule.
Citing this page:
Generalic, Eni. "Perioda." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table