electrolytic cell → elektrolitska ćelija
Electrolytic cell is an electrochemical cell that converts electrical energy into chemical energy. The chemical reactions do not occur spontaneously at the electrodes when they are connected through an external circuit. The reaction must be forced by applying an external electric current. It is used to store electrical energy in chemical form (rechargeable battery). It is also used to decompose or produce (synthesise) new chemicals by the application of electrical power. This process is called electrolysis, e.g., water can be decomposed into hydrogen gas and oxygen gas. The free energy change of the overall cell reaction is positive.
electromagnetic radiation spectrum → spektar elektromagnetskog zračenja
Wavelengths of electromagnetic waves can be shown with the help of electromagnetic radiation spectrum. Electromagnetic radiation spectrum is divided into several areas from γ-radiation of very short wavelengths and great energy to radio waves with wavelengths up to 1 000 m. The human eye can only see a narrow part of the electromagnetic spectrum - visible radiation.
electron → elektron
The electron is an elementary particle with a negative electric charge of (1.602 189 2±0.000 004 6)×10-19 C and a mass of 1/1837 that of a proton, equivalent to (9.109 534±0.000 047)×10-31 kg.
In 1897 the British physicist Joseph John (J.J.) Thomson (1856-1940) discovered the electron in a series of experiments designed to study the nature of electric discharge in a high-vacuum cathode-ray tube. Thomson interpreted the deflection of the rays by electrically charged plates and magnets as evidence of bodies much smaller than atoms that he calculated as having a very large value for the charge to mass ratio. Later he estimated the value of the charge itself.
Electrons are arranged in from one to seven shells around the nucleus; the maximum number of electrons in each shell is strictly limited by the laws of physics (2n2). The outer shells are not always filled: sodium has two electrons in the first shell (2×12 = 2), eight in the second (2×22 = 8), and only one in the third (2×32 = 18). A single electron in the outer shell may be attracted into an incomplete shell of another element, leaving the original atom with a net positive charge. Valence electrons are those that can be captured by or shared with another atom.
Electrons can be removed from the atoms by heat, light, electric energy, or bombardment with high-energy particles. Decaying radioactive nuclei spontaneously emit free electrons, called β particles.
electron configuration → elektronska konfiguracija
The electron configuration shows how many electrons there are in an atom or ion and their distribution along orbitals (see Table of electronic configuration of elements). Structure and all regularity in the periodic system depend upon electronic configuration of atoms of elements. Characteristics of elements mainly depend on electronic configuration of the outer shell. Refilling of the new electronic shell atoms of elements of similar electronic configuration emerge as well as in the previous shell, which adds up to periodicities of characteristics of elements.
electrophoresis → elektroforeza
Electrophoresis is a technique for the analysis and separation of colloids, based on the movement of charged colloidal particles in an electric field. The migration is toward electrodes of charge opposite to that of the particles. The rate of migration of the particles depends on the field, the charge on the particles, and on other factors, such as the size and shape of the particles.
Electrophoresis is important in the study of proteins. The acidity of the solution can be used to control the direction in which a protein moves upon electrophoresis.
electroplating → galvaniziranje
Electroplating (also called electrodeposition) is the deposition of a metallic coating onto an object by putting a negative charge onto the object and immersing it into a solution which contains a salt of the metal to be deposited. The metallic ions of the salt carry a positive charge and are attracted to the part. When they reach it, the negatively charged part provides the electrons to reduce the positively charged ions to metallic form.
Typically, a brass or nickel object is coated with a layer of silver by making use of electrolysis of a silver solution, using the object to be coated as the cathode. The anode consist of pure silver, and the cathode is the object to be plated. The electrolyte is a mixure of silver nitrate with potassium cyanide. The reactions are:
The cyanide ensures a low concentration of silver ions, a condition for providing the best plating results.
enantiomer → enantiomer
Enantiomers are a chiral molecule and its non-superposable mirror image. The two forms rotate the plane of polarised light by equal amounts in the opposite directions. Also called optical isomers.
enzyme → enzim
Enzyme is a protein that acts as a catalyst in biochemical reactions. Each enzyme is specific to a particular reaction or a group of similar reactions. Many require the association of certain nonprotein cofactors in order to function. The molecule undergoing a reaction (the substrate) binds to a specific active site on the enzyme molecule to form a short-lived intermediate: this greatly increases (by a factor of up to 1020) the rate at which the reaction proceeds to form the product.
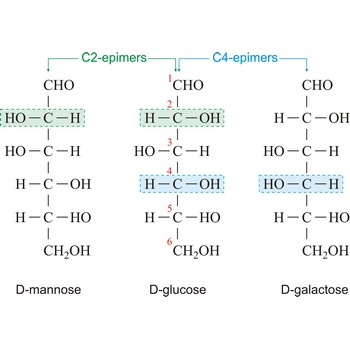
epimer → epimer
Epimers are diastereoisomers that have the opposite configuration at only one of two or more chiral centers present in the respective molecular entities. For example D-glucose and D-mannose, which differ only in the stereochemistry at C-2, are epimers, as are D-glucose and D-galactose (which differ at C-4).
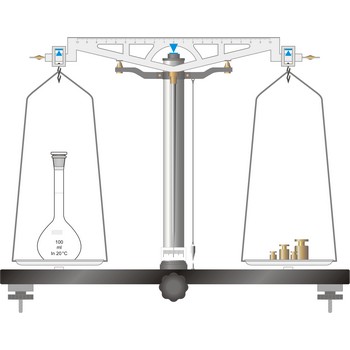
equal-arm balance → vaga s jednakim krakovima
The simplest type of balance, the equal-arm balance, is an application of a first class lever. The beam of the balance is supported on a central knife-edge, usually of agate, which rests upon a plane agate plate. The point of support is called the fulcrum. Two pans of equal weight are suspended from the beam, one at each end, at points equidistant from the fulcrum. A long pointer attached at right angles to the beam at the fulcrum indicates zero on a scale when the beam is at rest parallel to a level surface.
To prevent the knife-edge from becoming dull under the weight of the beam and pans the balance is equipped with a special device called an arrest. The arrest is operated by means of milled knob underneath the base plate in the middle and in front of the balance (sometimes the arrest knob is at one side of the balance).
The object to be weighed is placed on one pan, and standard weights are added to the other until the balance of the beam is established again. When not in use and during loading or unloading of the pans, the balance should be arrested.
Citing this page:
Generalic, Eni. "Perioda." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table