racemisation → racemizacija
Racemisation is a conversion, by heat or by chemical reaction, of an optically active compound into an optically inactive form which half of the optically active substance becomes its miror image (enantiomer).
white light → bijela svjetlost
White light is a mixture of lights of all colours. If white light is passed through a glass prism or an optical lattice, it is separated into several colours (the visible light spectrum).
poison → otrov
Poisons are substance, which upon contact or being introduced into an organism, impair or prevent normal metabolic processes from taking place, thus altering the normal functioning of organs or tissues.
Poisons are molecules or material that tends to collect on a catalyst surface, blocking access to active sites or destroying their activities.
Poisons are substance that can reduce a nuclear reaction by absorbing neutrons, thereby preventing more fission. If enough poisons are present in a reactor core, the chain reaction will die out.
polarimetry → polarimetrija
Polarimetry measures the overall turning of the flat of polarised light. It is used when analysing optically active substances and compounds.
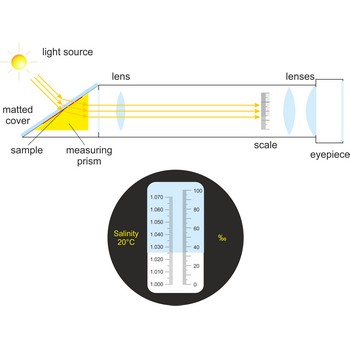
refractometer → refraktometar
Refractometer is an optical device used from measurement of refractive index. A refractometer takes advantage of the fact that light bends as it passes through different materials. It can be used to measure the salinity of water or the amount of sugar in fresh grapes. Refractometers are available with or without automatic temperature compensation (ATC).
When using a conventional saltwater refractometer, a sample is placed on an optical prism in the sample window. As light shines through the sample, it is bent according to the salinity of the water, and casts a shadow on the scale that is visible through the eyepiece. Salinity is read directly through the eyepiece.
stereoisomer → stereoizomer
Stereoisomers are compounds that have identical chemical constitution, but differ as regards the arrangement of the atoms or groups in space. Stereoisomers fall into two broad classes: optical isomers (enantiomers) and geometric isomers (cis-trans).
thallium → talij
Thallium was discovered by Sir William Crookes (England) in 1861. The origin of the name comes from the Greek word thallos meaning green twig or green shoot. It is soft grey metal that looks like lead. Tarnishes in moist air. Reacts in heated moist air and in acids. Compounds highly toxic by inhalation or ingestion. Cumulative effects. Thallium is found in iron pyrites. Also in crookesite, hutchinsonite and lorandite. Most is recovered from the by-products of lead and zinc refining. Its compounds are used in rat and ant poisons. Also for detecting infrared radiation.
Citing this page:
Generalic, Eni. "Optička aktivnost." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table