nonpolar molecule → nepolarna molekula
Nonpolar molecule is a molecule which has no separation of charge, so no positive or negative poles are formed. For example, the Cl2 molecule has no polar bonds (molecule with one type of atom), CH4 is a non-polar molecule (due to its symmetry). Nonpolar molecules do not dissolve in water as they cannot form hydrogen bonds (thus are hydrophobic) but do dissolve in lipids or fats (lipophilic).
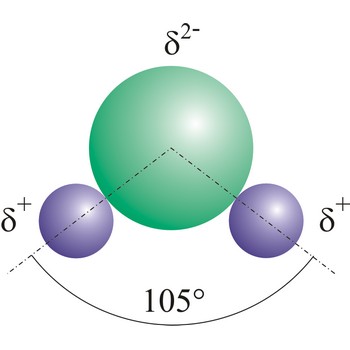
polar molecule → polarna molekula
Polar molecules are molecules at which centres of gravity of positive and negative charge are not in the same point.
relative molecular mass → relativna molekularna masa
Relative molecular mass (Mr) is the ratio of the average mass per molecule or specified entity of a substance to 1/12 of the mass of nuclide 12C. Also called molecular weight. It is equal to the sum of the relative atomic masses of all the atoms that comprise a molecule. For example
Mr(H2SO4) = 2·Ar(H) + Ar(S) + 4·Ar(O)
= 2·1.0079 + 32.066 + 4·15.999
= 2.0158 + 32.066 + 63.996
= 98.078
crystal → kristal
Crystal is a solid with a regular geometric shape, having a characteristic internal structure and enclosed by symmetrically arranged plane surfaces, intersecting at definite and characteristic angles. In crystals the particles (atoms, ions, or molecules) have a regular three-dimensional repeating arrangement in space. This is called the crystal structure. The crystal lattice is the arrangement of points in space at which the particles are positioned.
conformation → konformacija
Conformation is one of the very large numbers of possible spatial arrangements of atoms that can be interconverted by rotation about a single bond in a molecule. The conformation of a molecule is not fixed, though one or another shape may be more likely to occur. There are two extreme cases:
Staggered conformation (antiperiplanar) is a conformation about a carbon-carbon single bond in which the atoms on one carbon are as far apart as possible from the atoms on an adjacent carbon.
Eclipsed conformation (syn-periplanar) is a conformation about a carbon-carbon single bond in which the atoms on one carbon are as close as possible to the atoms on an adjacent carbon.
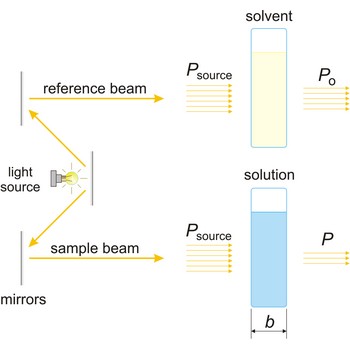
absorbance → apsorbancija
Absorbance (A) is a logarithm of the ratio of incident radiant power (Po) to transmitted radiant power (P) through a sample (excluding the effects on cell walls).
The absorption of light by a substance in a solution can be described mathematically by the Beer-Lambert law
where A is the absorbance at a given wavelength of light, ε is the molar absorbtivity or extinction coefficient (L mol-1 cm-1), unique to each molecule and varying with wavelength, b is the length of light path through the sample (cm), and c is the concentration of the compound in solution (mol L-1).
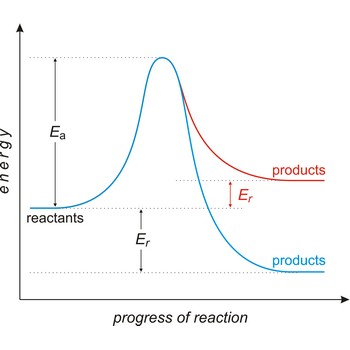
activation energy → energija aktivacije
Activation energy (Ea) is the energy that must be added to a system in order for a process to occur, even though the process may already be thermodynamically possible. In chemical kinetics, the activation energy is the height of the potential barrier separating the products and reactants. It determines the temperature dependence on the reaction rate.
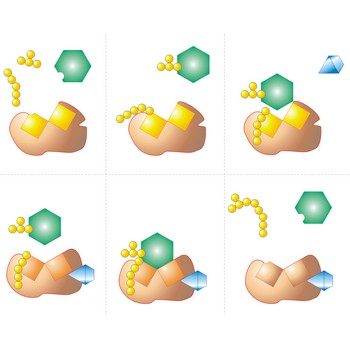
active site → aktivno mjesto
Active site is a pocket or crevice on an enzyme molecule that fits reactant molecules like a hand in a glove. The active site lowers the activation energy for reaction
AMU → AMU
AMU or atomic mass unit is a unit of mass used to express relative atomic masses. It is equal to 1/12 of the mass of an atom of the isotope carbon-12 and is equal to 1.66 033×10-27 kg. This unit superseded both the physical and a chemical mass unit based on oxygen-16 and is sometimes called the unified mass unit or the dalton.
Citing this page:
Generalic, Eni. "Oktaedarska geometrija molekule." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table