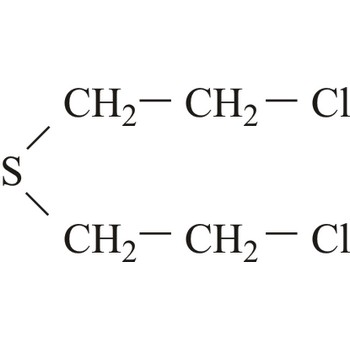mustard agent → plikavac
Mustard agents are usually classified as blistering agents owing to the similarity of the wounds caused by these substances resembling burns and blisters. However, since mustard agents also cause severe damage to the eyes, respiratory system and internal organs, they should preferably be described as blistering and tissue-injuring agents. Normal mustard agent (yperite), 1,1-thio-bis-[2-chloroethane], reacts with a large number of biological molecules. The effect of mustard agent is delayed and the first symptoms do not occur between 2-24 hours after exposure. At room temperature, mustard agent is a liquid with low volatility and is very stable during storage.
Nernst’s division law → Nernstov zakon razdjeljenja
Nernst’s division law states that a substance is divided between two solvents in a way that proportion of concentrations of that substance is at certain temperatures constant, under the condition that both solvents are in the same molecular state. Division coefficient is a proportion of substance concentration in solvents A i B at a defined temperature.
Appearance of division is used for substance extraction.
Newman’s projection → Newmanova projekcija
Newman’s projection is an image which we get when we observe a model of ethane molecule in C-C bond direction.
non-metal → nemetal
Non-metals are defined as elements that are not metals.
Their physical properties generally include:
- They are poor conductors.
- They are brittle, not ductile in their solid state.
- They show no metallic lustre.
- They may be transparent or translucent.
- They have low density.
- They form molecules which consists of atoms covalently bonded; the noble gases are monoatomic.
Their chemical properties are generally:
- They usually have four to eight valence electrons.
- They have high electron affinities (except the noble gases)
- They are good oxidising agents (except the noble gases)
- They have hydroxides which are acidic (except the noble gases)
- They are electronegative.
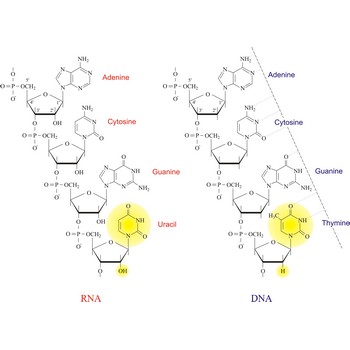
nucleic acid → nukleinska kiselina
Nucleic acids are a complex, high-molecular-weight biochemical macromolecules composed of nucleotide chains that convey genetic information. The most common nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Each nucleic acid chain is composed of subunits called nucleotides, each containing a sugar, a phosphate group, and nitrogenous base. DNA was first discovered in 1869 by the Swiss biochemist Friedrich Miescher (1844-1895).
Both DNA and RNA contain the two major purine bases adenine (A) and guanine (G) and one of the major pyrimidines, cytosine (C). Of the other two pyrimidines, thymine (T) is found in DNA and uracil (U) is found in RNA. There are two major pentoses in nucleic acids:2'-deoxy-D-ribose in DNA and D-ribose in RNA.
Nucleotides are linked together in both DNA and RNA in a polymeric fashion via covalent bonds. These bonds exist through phosphate-group bridges in which the 5' hydroxyl group of one nucleotide unit is joined to the 3' hydroxyl group of the next nucleotide. RNA is usually a single-stranded molecule, whereas DNA is usually double-stranded.
octahedron → oktaedar
Octahedron is a three-dimensional geometric figure having eight triangular sides.
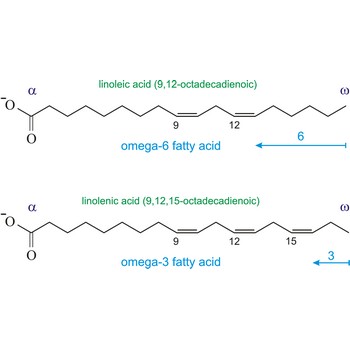
omega-3 fatty acids → omega-3 masne kiseline
Omega-3 fatty acids are polyunsaturated fatty acids, meaning they contain more than one double bond. The name omega-3 indicates that the first double bond occurs on the third carbon atom (n-3) from the methyl (-CH3) end of the molecule (omega position). The three main omega-3 fatty acids are alpha-linolenic acid (ALA, 18:3n-3), eicosapentaenoic acid (EPA, 20:5n-3), and docosahexaenoic acid (DHA, 22:6n-3). ALA comes from plants. EPA and DHA come from fish.
Similarly, the first double bond in omega-6 fatty acids is located between the sixth and seventh carbon atom (n-6) from the methyl end of the fatty acid (omega end).
optical activity → optička aktivnost
Optical activity is the ability of a chiral molecule to rotate the plane of plane-polairzed light. Molecules of an optically active substance cannot be superimposed on their own mirror images, just as your left hand cannot be superimposed on your right when both are held palm-down.
optically active matter → optički aktivna tvar
Optically active matter, by polarized light passing through it, turns the flat of polarized light leftwards or rightwards.
orbital → orbitala
Orbital is the area in space about an atom or molecule in which the probability of finding an electron is greatest.
The possible atomic orbitals correspond to subshells of the atom. Thus there is one s-orbital for each shell (orbital quantum number l = 0). There are three p-orbitals (corresponding to the three values of l) and five d-orbitals. The shapes of orbitals depend on the value of l.
Citing this page:
Generalic, Eni. "Oktaedarska geometrija molekule." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table