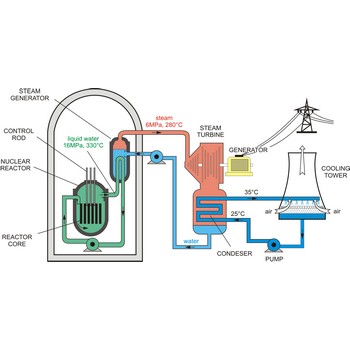
nuclear reactor → nuklearni reaktor
Nuclear reactor is an assembly of fissionable material (uranium-235 or plutonium-239) designed to produce a sustained and controllable chain reaction for the generation of electric power.
The essential components of a nuclear reactor are:
- The core, metal rods containing enough fissionable material to maintain a chain reaction at the necessary power level (as much as 50 t of uranium may be required).
- A source of neutrons to initiate the reaction (such as a mixture of polonium and beryllium)
- A moderator to reduce the energy of fast neutrons for more efficient fission (material such as graphite, beryllium, heavy water, and light water are used)
- A coolant to remove the fission-generated heat (water, sodium, helium, and nitrogen may be used)
- A control system such as rods of boron or cadmium that have high capture cross sections (to absorb neutrons)
- Adequate shielding, remote-control equipment, and appropriate instrumentation are essential for personnel safety and efficient operation.
nuclear magnetic resonance → nuklearna magnetska rezonancija
Nuclear magnetic resonance (NMR) is a type of radio-frequency spectroscopy based on the magnetic field generated by the spinning of electrically charged atomic nuclei. This nuclear magnetic field is caused to interact with a very large (1 T - 5 T) magnetic field of the instrument magnet. NMR techniques have been applied to studies of electron densities and chemical bonding and have become a fundamental research tool for structure determinations in organic chemistry.
actinides → aktinoidi
Actinides (actinons or actinoids) are the fourteen elements from thorium to lawrencium inclusive, which follow actinium in the periodic table. The position of actinium is somewhat equivocal and, although not itself an actinide, it is often included with them for comparative purpose. The series includes the following elements: thorium (Th), protactinium (Pa), uranium (U), neptunium (Np), plutonium (Pu), amercium (Am), curium (Cm), berkelium (Bk), californium (Cf), einsteinium (Es), fermium (Fm), mendelevium (Md), nobelium (No) and lawrencium (Lr). Every known isotope of the actinide elements is radioactive. Traces of Pa, Np and Pu are consequently found, but only Th and U occur naturally to any useful extent.
americium → americij
Americium was discovered by Glenn T. Seaborg, Ralph A. James, Stanley G. Thompson and Albert Ghiorso (USA) in 1944. Named for the American continent. It is silvery-white, artificially produced radioactive metal. Americium was produced by bombarding plutonium with neutrons. Americium-241 is currently used in smoke detectors.
artificial radioactive isotope → umjetni radioaktivni izotop
Artificial radioactive isotopes are formed when an atom is bombed with an accelerator or exposing it to slow moving neutrons in a nuclear reactor. In this way isotopes (radionuclides) are obtained which are non-existent in nature because of their unstability and radioactive transition into stable isotopes. Most important radioactive isotopes are:
Radioactive isotope of cobalt is formed when ordinary metal cobalt is bombed with neutrons in a nuclear reactor.
Radioactive isotope of phosphorus is formed when ordinary phosphorus is bombed with deuterons produced in cyclotron.
radioactive isotope of carbon is formed when a nitrogen is bombed with slow moving neutrons in a nuclear reactor. It is mostly used as a radioactive indicator.
artificial radioactivity → umjetna radioaktivnost
Artificial radioactivity is a creation, with the help of an accelerator or in the nuclear reactor, of isotopes (radionuclides) which are found in nature because they are unstable and by radioactive conversion they are converted to stable isotopes.
berkelium → berkelij
Berkelium was discovered by Stanley G. Thompson, Albert Ghiorso and Glenn T. Seaborg (USA) in 1949. Named after Berkeley, a city in California, home of the University of California, USA. It is synthetic radioactive metal. Berkelium was made by bombarding americium with alpha particles.
beryllium → berilij
Beryllium was discovered by Friedrich Wöhler (Germany) and independently by A. B. Bussy (France) in 1828. The origin of the name comes from the Greek word beryllos meaning mineral beryl; also called glucinium from the Greek word glykys meaning sweet. It is steel-grey metal. It resists attack by concentrated nitric acid, has excellent thermal conductivity and is nonmagnetic. At ordinary temperatures, it resists oxidation in air. Beryllium and its salts are toxic and should be handled with the greatest of care. Beryllium is found mostly in minerals like beryl [AlBe3(Si6O18)] and chrysoberyl (Al2BeO4). Pure beryllium is obtained by chemically reducing beryl mineral. Also by electrolysis of beryllium chloride. Its ability to absorb large amounts of heat makes it useful in spacecraft, missiles, aircraft, etc. Emeralds are beryl crystals with chromium traces giving them their green colour.
bismuth → bizmut
Bismuth was discovered by Claude Geoffroy (France) in 1753. The origin of the name comes from the German words Weisse Masse meaning white mass; now spelled wismut and bisemutum. It is hard, brittle, steel-grey metal with a pink tint. Stable in oxygen and water. Dissolves in concentrated nitric acid. Bismuth can be found free in nature and in minerals like bismuthine (Bi2S3) and in bismuth ochre (Bi2O3) Main use is in pharmaceuticals and low melting point alloys used as fuses.
cadmium → kadmij
Cadmium was discovered by Friedrich Strohmeyer (Germany) in 1817. The origin of the name comes from the Latin word cadmia meaning calamine (zinc carbonate, ZnCO3), or from the Greek word kadmeia with the same meaning. It is soft, malleable, blue-white metal. Tarnishes in air, soluble in acids, insoluble in alkalis. Boiling cadmium gives off a weird, yellow-colored vapour that is poisonous. Cadmium can cause a variety of health problems, including kidney failure and high blood pressure. Cadmium is obtained as a by product of zinc refining. The mayor use of cadmium is in electroplating of steel to protect it from corrosion. Also used to make nickel-cadmium batteries. The ability of cadmium to adsorb neutrons has made it of great importance in the design of nuclear reactors. Its compounds are found in paint pigments and a wide variety of intense colours.
Citing this page:
Generalic, Eni. "Nuklearni reaktor." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table