vanadium → vanadij
Vanadium was discovered by A. M. del Rio (Spain) in 1801 and rediscovered by Nils Sefstrom (Sweden) in 1830. Named after Vanadis, the goddess of beauty in Scandinavian mythology. It is soft, ductile, silvery-white metal. Resistant to corrosion by moisture, air and most acids and alkalis at room temperature. Exposed surfaces form oxide coating. Reacts with concentrated acids. Vanadium is found in the minerals patronite (VS4), vanadinite [Pb5(VO4)3Cl] and carnotite [K2(UO2)2(VO4)2·3H2O]. Pure metal produced by heating with C and Cl to produce VCl3 which is heated with Mg in Ar atmosphere. It is mixed with other metals to make very strong and durable alloys. Vanadium pentoxide (V2O5) is used as a catalyst, dye and fixer-fixer.
Volta, Alessandro → Volta, Alessandro
The Italian physicist Alessandro Giuseppe Antonio Anastasio Volta (1745-1827) was the inventor of the voltaic pile, the first electric battery (1800). In 1775 he invented the electrophorus, a device that, once electrically charged by having been rubbed, could transfer charge to other objects. Between 1776 and 1778, Volta discovered and isolated methane gas (CH4). The electrical unit known as the volt was named in his honor.
water hardness → tvrdoća vode
Hardness is defined as the concentrations of calcium and magnesium ions expressed in terms of calcium carbonate. These minerals in water can cause some everyday problems. They react with soap and produce a deposit called soap curd that remains on the skin and clothes and, because it is insoluble and sticky, cannot be removed by rinsing.
Hard water may also shorten the life of plumbing and water heaters. When water containing calcium carbonate is heated, a hard scale is formed that can plug pipes and coat heating elements. Scale is also a poor heat conductor. With increased deposits on the unit, heat is not transmitted to the water fast enough and overheating of the metal causes failure. Build-up of deposits will also reduce the efficiency of the heating unit, increasing the cost of fuel.
There are two types of water hardness, temporary and permanent.
Temporary Hardness is due to the bicarbonate ion, HCO3-, being present in the water. This type of hardness can be removed by boiling the water to expel the CO2, as indicated by the following equation:
Permanent hardness is due to calcium and magnesium nitrates, sulphates, and chlorides etc. This type of hardness cannot be eliminated by boiling.
| Water supply classification | |
|---|---|
| Hardness | Concentration of Calcium carbonate (mg/L) |
| Soft Water | 0 to 75 |
| Medium Hard Water | 75 to 150 |
| Hard Water | 150 to 300 |
| Very Hard Water | over 300 |
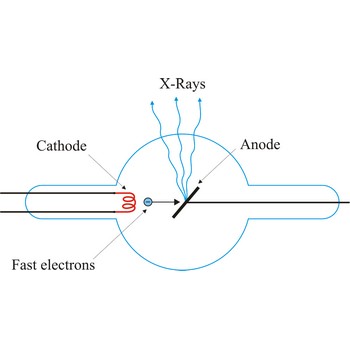
X-ray tube → rendgenska cijev
X-ray tube is a cathode ray tube that focuses energetic streams of electrons on a metal target, causing the metal to emit X-rays. The basic principle of the X-ray tube has not changed significantly since Roentgen's 1895 discovery. Current applied to a metal cathode (about 50 000 V) produces free electrons. The X-rays are produced when the rapidly moving electrons are suddenly stopped as they strike the metal target of the tube.
ytterbium → iterbij
Ytterbium was discovered by Jean de Marignac (France) in 1878. Named after Ytterby, a village in Sweden. It is silvery, lustrous, malleable and ductile metal. Oxidizes slowly in air. Reacts with water. Flammable dust. Ytterbium is found in minerals such as yttria, monazite, gadolinite and xenotime. Used in metallurgical and chemical experiments.
yttrium → itrij
Yttrium was discovered by Carl Gustaf Mosander (Sweden) in 1843. Named after Ytterby, a village in Sweden. It is silvery, ductile, fairly reactive metal. Exposed surfaces form oxide film. Easily combustible, reacts with oxygen in water to release hydrogen. Yttrium is found in minerals such as monazite, xenotime and yttria. Combined with europium to make red phosphors for colour TV’s. Yttrium oxide and iron oxide combine to form a crystal garnet used in radar.
zinc → cink
Zinc was discovered by Andreas Marggraf (Germany) in 1746. The origin of the name comes from the German word zink. It is bluish-silver, ductile metal. Reacts with alkalis and acids. Tarnishes in air. Zinc is found in the minerals zinc blende (sphalerite) (ZnS), calamine, franklinite, smithsonite (ZnCO3), willemite and zincite (ZnO). Used to coat other metal (galvanizing) to protect them from rusting. Although some 90 % of the zinc is used for galvanizing steel. Zinc metal is used in the common dry-cell battery. Also used in alloys such as brass, bronze. Zinc compounds are also used in the manufacture of paints, cosmetics, plastics, electronic devices, and other products.
zirconium → cirkonij
Zirconium was discovered by Martin Heinrich Klaproth (Germany) in 1789. The origin of the name comes from the Arabic word zargun meaning gold colour. It is grey-white, lustrous, corrosion-resistant metal. Exposed surfaces form oxide protective film. Zirconium is found in many minerals such as zircon and baddeleyite. Used in alloys such as zircaloy this is used in nuclear applications since it does not readily absorb neutrons. Also baddeleyite is used in lab crucibles. Used in high-performance pumps and valves. Clear zircon (ZrSiO4) is a popular gemstone.
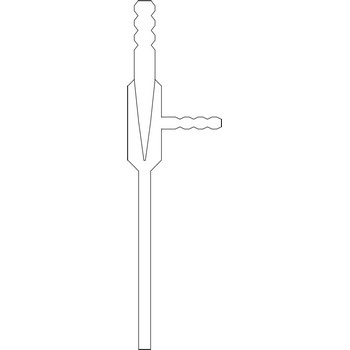
water jet vacuum pump → vodena sisaljka
The water jet vacuum pump or vacuum aspirator is one of the most popular devices that produces vacuum in laboratories. The rapid flow of water through the device creates a vacuum in a side-arm that is connected to a vacuum application such a Buchner flask. The water jet vacuum pump creates a vacuum by means of Venturi effect named after the Italian physicist Giovanni Battista Venturi (1746–1822). The Venturi effect is the reduction in fluid pressure that results when a fluid flows through a constricted section of pipe. Water jet pumps are manufactured of glass, plastic or metal, depending on the conditions in which they are used.
Citing this page:
Generalic, Eni. "Meta položaj." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table